A phase 2 trial of gemcitabine plus toripalimab for cisplatin-ineligible patients with recurrent or metastatic nasopharyngeal carcinoma
- PMID: 39413735
- PMCID: PMC11514602
- DOI: 10.1016/j.xcrm.2024.101779
A phase 2 trial of gemcitabine plus toripalimab for cisplatin-ineligible patients with recurrent or metastatic nasopharyngeal carcinoma
Abstract
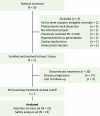
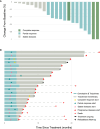
Cisplatin is a cornerstone chemotherapy for nasopharyngeal carcinoma (NPC); however, certain patients are ineligible for cisplatin-based regimens. This phase 2 trial (NCT04405622) evaluated the efficacy and safety of gemcitabine and toripalimab in previously untreated patients with recurrent or metastatic NPC who were either ineligible for cisplatin or had experienced severe adverse events from prior cisplatin-based treatments. Patients received gemcitabine (1,000 mg/m2) and toripalimab (240 mg) every three weeks for six cycles, followed by toripalimab monotherapy for up to two years. The primary endpoint was the incidence of grade ≥3 adverse events, while secondary endpoints included objective response rate (ORR) and overall survival (OS). Of 30 screened patients, 21 were enrolled. No treatment-related fatalities occurred, with the most frequent adverse events being headache and nausea. The ORR was 61.9%, coupled with a disease control rate of 100%. Overall, gemcitabine plus toripalimab demonstrated low toxicity and promising efficacy for this specific patient cohort.
Keywords: PD-1; cisplatin; first-line; gemcitabine; ineligible; nasopharyngeal carcinoma.
Copyright © 2024 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures


References
-
- Chen Y.P., Ismaila N., Chua M.L.K., Colevas A.D., Haddad R., Huang S.H., Wee J.T.S., Whitley A.C., Yi J.L., Yom S.S., et al. Chemotherapy in Combination With Radiotherapy for Definitive-Intent Treatment of Stage II-IVA Nasopharyngeal Carcinoma: CSCO and ASCO Guideline. J. Clin. Oncol. 2021;39:840–859. doi: 10.1200/jco.20.03237. - DOI - PubMed
-
- Sadeghi S., Groshen S.G., Tsao-Wei D.D., Parikh R., Mortazavi A., Dorff T.B., Kefauver C., Hoimes C., Doyle L., Quinn D.I., et al. Phase II California Cancer Consortium Trial of Gemcitabine-Eribulin Combination in Cisplatin-Ineligible Patients With Metastatic Urothelial Carcinoma: Final Report (NCI-9653) J. Clin. Oncol. 2019;37:2682–2688. doi: 10.1200/jco.19.00861. - DOI - PMC - PubMed
-
- Holmsten K., Jensen N.V., Mouritsen L.S., Jonsson E., Mellnert C., Agerbæk M., Nilsson C., Moe M., Carus A., Öfverholm E., et al. Vinflunine/gemcitabine versus carboplatin/gemcitabine as first-line treatment in cisplatin-ineligible patients with advanced urothelial carcinoma: A randomised phase II trial (VINGEM) Eur. J. Cancer. 2020;127:173–182. doi: 10.1016/j.ejca.2019.08.033. - DOI - PubMed
-
- Balar A.V., Galsky M.D., Rosenberg J.E., Powles T., Petrylak D.P., Bellmunt J., Loriot Y., Necchi A., Hoffman-Censits J., Perez-Gracia J.L., et al. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial. Lancet (London, England) 2017;389:67–76. doi: 10.1016/s0140-6736(16)32455-2. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

