Modeling lung adenocarcinoma metastases using patient-derived organoids
- PMID: 39413736
- PMCID: PMC11513837
- DOI: 10.1016/j.xcrm.2024.101777
Modeling lung adenocarcinoma metastases using patient-derived organoids
Abstract
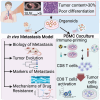
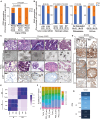
Approximately 50% of patients with surgically resected early-stage lung cancer develop distant metastasis. At present, there is no in vivo metastasis model to investigate the biology of human lung cancer metastases. Using well-characterized lung adenocarcinoma (LUAD) patient-derived organoids (PDOs), we establish an in vivo metastasis model that preserves the biologic features of human metastases. Results of whole-genome and RNA sequencing establish that our in vivo PDO metastasis model can be used to study clonality and tumor evolution and to identify biomarkers related to organotropism. Investigation of the response of KRASG12C PDOs to sotorasib demonstrates that the model can examine the efficacy of treatments to suppress metastasis and identify mechanisms of drug resistance. Finally, our PDO model cocultured with autologous peripheral blood mononuclear cells can potentially be used to determine the optimal immune-priming strategy for individual patients with LUAD.
Keywords: coculture; drug resistance; immune priming; in vivo LUAD metastasis; lung adenocarcinoma; metastasis marker; patient-derived organoids; tumor evolution.
Copyright © 2024 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests G.R. has financial relationships with Scanlan, AstraZeneca, and Medtronic. S.S. is a member of the AstraZeneca Advisory Board. J.M.I. has stock ownership in LumaCyte and is a consultant/advisory board member for Roche Genentech. M.J.B. is a consultant for AstraZeneca, Iovance Biotherapeutics, and Intuitive Surgical and receives research support from Obsidian Therapeutics. B.T.L. has served as an uncompensated advisor and consultant to Amgen, AstraZeneca, Boehringer Ingelheim, Bolt Biotherapeutics, Daiichi Sankyo, Genentech, and Lilly; has received research grants (institutional) from Amgen, AstraZeneca, Bolt Biotherapeutics, Daiichi Sankyo, Genentech, Hengrui USA, and Lilly; has received academic travel support from Amgen, Jiangsu Hengrui Medicine, and MORE Health; and has intellectual property rights as a book author at Karger Publishers and Shanghai Jiao Tong University Press. M.H.B. receives royalties from Globus Medical and DePuy Synthes. P.S.A. declares research funding from Atara Biotherapeutics; is a scientific advisory board member and consultant for ATARA Biotherapeutics, Bayer, Bio4T2, Carisma Therapeutics, Imugene, ImmPACT Bio, Johnson & Johnson, Orion, and Outpace Bio; has patents, royalties, and intellectual property on mesothelin-targeted chimeric antigen receptor and other T cell therapies, which have been licensed to Atara Biotherapeutics; and has an issued patent method for detection of cancer cells using virus and pending patent applications on PD-1 dominant negative receptor, a wireless pulse-oximetry device, and an ex vivo malignant pleural effusion culture system. MSK has licensed intellectual property related to mesothelin-targeted chimeric antigen receptors and T cell therapies to Atara Biotherapeutics and has associated financial interests. D.R.J. serves on a clinical trial steering committee for AstraZeneca and has research grant support from Merck.
Figures



References
-
- Drilon A., Wang L., Arcila M.E., Balasubramanian S., Greenbowe J.R., Ross J.S., Stephens P., Lipson D., Miller V.A., Kris M.G., et al. Broad, hybrid capture-based next-generation sequencing identifies actionable genomic alterations in lung adenocarcinomas otherwise negative for such alterations by other genomic testing approaches. Clin. Cancer Res. 2015;21:3631–3639. doi: 10.1158/1078-0432.CCR-14-2683. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

