Immune effector cell-associated enterocolitis following chimeric antigen receptor T-cell therapy in multiple myeloma
- PMID: 39414769
- PMCID: PMC11484697
- DOI: 10.1038/s41408-024-01167-8
Immune effector cell-associated enterocolitis following chimeric antigen receptor T-cell therapy in multiple myeloma
Abstract
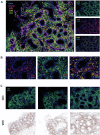
We report 14 cases of immune effector cell (IEC)-associated enterocolitis following chimeric antigen receptor T-cell (CAR-T) therapy in multiple myeloma, with a 1.2% incidence overall (0.2% for idecabtagene vicleucel and 2.2% for ciltacabtagene autoleucel). Patients developed acute-onset symptoms (typically non-bloody Grade 3+ diarrhea) with negative infectious workup beginning a median of 92.5 days (range: 22-210 days) after CAR-T therapy and a median of 85 days after cytokine release syndrome resolution. Gut biopsies uniformly demonstrated inflammation, including intra-epithelial lymphocytosis and villous blunting. In one case where CAR-specific immunofluorescence stains were available, CAR T-cell presence was confirmed within the lamina propria. Systemic corticosteroids were initiated in 10 patients (71%) a median of 25.5 days following symptom onset, with symptom improvement in 40%. Subsequent infliximab or vedolizumab led to improvement in 50% and 33% of corticosteroid-refractory patients, respectively. Five patients (36%) have died from bowel perforation or treatment-emergent sepsis. In conclusion, IEC-associated enterocolitis is a distinct but rare complication of CAR-T therapy typically beginning 1-3 months after infusion. Thorough diagnostic workup is essential, including evaluation for potential T-cell malignancies. The early use of infliximab or vedolizumab may potentially hasten symptom resolution and lower reliance on high-dose corticosteroids during the post-CAR-T period.
© 2024. The Author(s).
Conflict of interest statement
RB reports consulting: Adaptive Biotech, BMS, Caribou Biosciences, Genentech, GSK, Janssen, Karyopharm, Legend Biotech, Pfizer, Sanofi, SparkCures; Research: Abbvie, BMS, Janssen, Novartis, Pack Health, Prothena, Sanofi. RF reports consulting: AbbVie, Adaptive, Amgen, Apple, BMS/Celgene, GSK, Janssen, Karyopharm, Pfizer, RA Capital, Regeneron, Sanofi; Scientific Advisory Board: Caris Life Sciences; Board of Directors: Antengene; Patents for certain FISH tests in MM. CF reports consulting: Janssen; research: Regeneron, Janssen; stock ownership: Affimed. PMV reports consulting: Abbvie, Astra Zeneca, BMS, GSK, Janssen, Karyopharm, Lava Therapeutics, Regeneron, Sanofi; research: Abbvie, Janssen, Regeneron. SDR reports honoraria: Janssen, BMS; steering committee involvement, Gracell Therapeutics, BMS; research support, Janssen, BMS, C4 Therapeutics, Gracell Therapeutics, Heidelberg Pharma; consulting: Genentech, Janssen, BMS. JYS reports consulting: Kite, Immpact Bio. LL reports consulting: Allogene, Sanofi. SFP reports Consulting: Cartography Biosciences; Scientific Advisory Board: Leica Biosystems. JLW reports consulting: Adaptive. AJC reports consulting: BMS, Adaptive; Research: Adaptive Biotechnologies, Harpoon, Nektar, BMS, Janssen, Sanofi, AbbVie. DWS reports consulting: GlaxoSmithKline, Janssen, Sanofi, Abbvie, Bristol Myer Squibb, Pfizer, Bioline, Arcellx, AstraZeneca, Genentech; Research: Gilead, Pfizer, Janssen, Bioline, GlaxoSmithKline, Sanofi, Amgen, Cantex, Arcellx, Roche; Steering Committee: Janssen; Data safety and monitoring: Karyopharm, and Independent Review Committee: Parexel. FLL reports a consulting or advisory role for Allogene, Amgen, Bluebird Bio, Bristol Myers Squibb/Celgene, Calibr, Cellular Biomedicine Group, Cowen, ecoR1, Emerging Therapy Solutions Gerson Lehman Group, GammaDelta Therapeutics, Iovance, Janssen, Kite, a Gilead Company, Legend Biotech, Novartis, Umoja, and Wugen; research funding from Allogene, Kite, and Novartis; and patents, royalties, other intellectual property in the field of cellular immunotherapy. P.V reports consulting or advisory role for Abbvie, AstraZeneca, Karyopharm Therapeutics, Bristol Myers Squibb, Lava Therapeutics, Sanofi, Janssen, GlaxoSmithKline; Research funding from Abbvie, Janssen, GlaxoSmithKline, and TeneoBio; Travel, accommodations, and expenses from Sanofi. DKH reports consulting: BMS, Janssen, legend Biotech, Pfizer, Karyopharm; Research: BMS, Karyopharm, Adaptive Biotechnologies, and Pentecost Myeloma Research Center. The remaining authors have no interests to disclose.
Figures

References
-
- Rodriguez-Otero P, Ailawadhi S, Arnulf B, Patel K, Cavo M, Nooka AK, et al. Ide-cel or Standard Regimens in Relapsed and Refractory Multiple Myeloma. N. Engl J Med. 2023;388:1002–14. - PubMed
-
- San-Miguel J, Dhakal B, Yong K, Spencer A, Anguille S, Mateos MV, et al. Cilta-cel or Standard Care in Lenalidomide-Refractory Multiple Myeloma. N. Engl J Med. 2023;389:335–47. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

