Venetoclax triggers sublethal apoptotic signaling in venetoclax-resistant acute myeloid leukemia cells and induces vulnerability to PARP inhibition and azacitidine
- PMID: 39414773
- PMCID: PMC11484809
- DOI: 10.1038/s41419-024-07140-4
Venetoclax triggers sublethal apoptotic signaling in venetoclax-resistant acute myeloid leukemia cells and induces vulnerability to PARP inhibition and azacitidine
Abstract
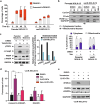
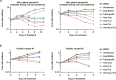
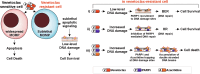
Venetoclax plus azacitidine treatment is clinically beneficial for elderly and unfit acute myeloid leukemia (AML) patients. However, the treatment is rarely curative, and relapse due to resistant disease eventually emerges. Since no current clinically feasible treatments are known to be effective at the state of acquired venetoclax resistance, this is becoming a major challenge in AML treatment. Studying venetoclax-resistant AML cell lines, we observed that venetoclax induced sublethal apoptotic signaling and DNA damage even though cell survival and growth were unaffected. This effect could be due to venetoclax inducing a sublethal degree of mitochondrial outer membrane permeabilization. Based on these results, we hypothesized that the sublethal apoptotic signaling induced by venetoclax could constitute a vulnerability in venetoclax-resistant AML cells. This was supported by screens with a broad collection of drugs, where we observed a synergistic effect between venetoclax and PARP inhibition in venetoclax-resistant cells. Additionally, the venetoclax-PARP inhibitor combination prevented the acquisition of venetoclax resistance in treatment naïve AML cell lines. Furthermore, the addition of azacitidine to the venetoclax-PARP inhibitor combination enhanced venetoclax induced DNA damage and exhibited exceptional sensitivity and long-term responses in the venetoclax-resistant AML cell lines and samples from AML patients that had clinically relapsed under venetoclax-azacitidine therapy. In conclusion, we mechanistically identify a new vulnerability in acquired venetoclax-resistant AML cells and identify PARP inhibition as a potential therapeutic approach to overcome acquired venetoclax resistance in AML.
© 2024. The Author(s).
Conflict of interest statement
MKo reports personal fees from Astellas Pharma, AbbVie, Bristol-Myers Squibb, Faron, Jazz, Pharmaceuticals, Novartis, and Pfizer and research funding from AbbVie outside the submitted work. CAH has received honoraria from Amgen, personal fees from Autolus, and research funding from Celgene/BMS, Kronos Bio, Novartis, Oncopeptides, Orion Pharma, WntReseach, Zentalis Pharmaceuticals, and the IMI2 projects HARMONY and HARMONY PLUS unrelated to this study. MCK is currently an employee of Ascendis Pharma.
Figures



References
-
- DiNardo CD, Jonas BA, Pullarkat V, Thirman MJ, Garcia JS, Wei AH, et al. Azacitidine and venetoclax in previously untreated acute myeloid leukemia. N. Engl J Med. 2020;383:617–29. - PubMed
-
- Bock FJ, Tait SWG. Mitochondria as multifaceted regulators of cell death. Nat Rev Mol Cell Biol. 2020;21:85–100. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

