A self-adjuvanted VLPs-based Covid-19 vaccine proven versatile, safe, and highly protective
- PMID: 39414952
- PMCID: PMC11484777
- DOI: 10.1038/s41598-024-76163-w
A self-adjuvanted VLPs-based Covid-19 vaccine proven versatile, safe, and highly protective
Abstract
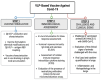
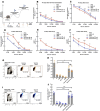
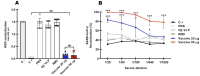
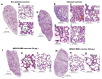
Vaccination has played a critical role in mitigating COVID-19. Despite the availability of licensed vaccines, there remains a pressing need for improved vaccine platforms that provide high protection, safety, and versatility, while also reducing vaccine costs. In response to these challenges, our aim is to create a self-adjuvanted vaccine against SARS-CoV-2, utilizing Virus-Like Particles (VLPs) as the foundation. To achieve this, we produced bacteriophage (Qβ) VLPs in a prokaryotic system and purified them using a rapid and cost-effective strategy involving organic solvents. This method aims to solubilize lipids and components of the cell membrane to eliminate endotoxins present in bacterial samples. For vaccine formulation, Receptor Binding Domain (RBD) antigens were conjugated using chemical crosslinkers, a process compatible with Good Manufacturing Practice (GMP) standards. Transmission Electron Microscopy (TEM) confirmed the expected folding and spatial configuration of the QβVLPs vaccine. Additionally, vaccine formulation assessment involved SDS-PAGE stained with Coomassie Brilliant Blue, Western blotting, and stereomicroscopic experiments. In vitro and in vivo evaluations of the vaccine formulation were conducted to assess its capacity to induce a protective immune response without causing side effects. Vaccine doses of 20 µg and 50 µg stimulated the production of neutralizing antibodies. In in vivo testing, the group of animals vaccinated with 50 µg of vaccine formulation provided complete protection against virus infection, maintaining stable body weight without showing signs of disease. In conclusion, the QβVLPs-RBD vaccine has proven to be effective and safe, eliminating the necessity for supplementary adjuvants and offering a financially feasible approach. Moreover, this vaccine platform demonstrates flexibility in targeting Variants of Concern (VOCs) via established conjugation protocols with VLPs.
Keywords: COVID-19; SARS-CoV-2; VLPs platform; Vaccine.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Kumar, A. et al. SARS-CoV-2-specific virulence factors in COVID-19. J. Med. Virol. 93 (3), 1343–1350. 10.1002/jmv.26615 (2021). Epub 2020 Nov 1. PMID: 33085084. - PubMed
-
- World Health Organization. WHO Coronavirus (COVID-19) Dashboard. https://covid19.who.int/
MeSH terms
Substances
Grants and funding
- 2020/09404-0 (Post-doc Scholarship)/Fundação de Amparo à Pesquisa do Estado de São Paulo
- AL: 2021/03102-5 (PhD Scholarship/Fundação de Amparo à Pesquisa do Estado de São Paulo
- NC 2021/03508-1/Fundação de Amparo à Pesquisa do Estado de São Paulo
- WAPS 2021/08468-8 (PhD Scholarship)/Fundação de Amparo à Pesquisa do Estado de São Paulo
- GC-M: 2019/14526-0, 2020/04667-3/Fundação de Amparo à Pesquisa do Estado de São Paulo
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

