Obesity-induced extracellular vesicles proteins drive the endometrial cancer pathogenesis: therapeutic potential of HO-3867 and Metformin
- PMID: 39414985
- PMCID: PMC11602708
- DOI: 10.1038/s41388-024-03182-2
Obesity-induced extracellular vesicles proteins drive the endometrial cancer pathogenesis: therapeutic potential of HO-3867 and Metformin
Abstract
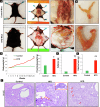
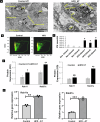
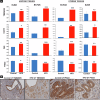
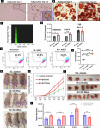
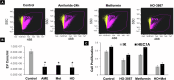
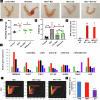
Endometrial cancer (EC) is the leading gynecologic malignancy in the United States with obesity implicated in 57% of cases. This research investigates the molecular complexities of extracellular vesicles (EV) secretion as carriers of oncogenic protein and their involvement in obesity-mediated EC. An understanding of these mechanisms is pivotal for unraveling pathways relevant to obesity-associated EC, thereby guiding the development of innovative prevention and treatment strategies. Our exploration revealed a significant increase in EV secretion carrying oncogenic proteins (TMEM205, STAT5, and FAS) in adipose and uterine tissues/serum samples from obese EC patients compared to control (without cancer). We identified alterations in EV-regulating proteins (Rab7, Rab11, and Rab27a) in obesity-mediated EC patients, adipose/uterine tissues, and serum samples. Through a 24-week analysis of the effects of a 45% kcal high-fat diet (HFD) on mice, we observed increased body weight, increased adipose tissue, enlarged uterine horns, and increased inflammation in the HFD group. This correlated with elevated levels of EV secretion and increased expression of oncogenic proteins TMEM205, FAS, and STAT5 and downregulation of the tumor suppressor gene PIAS3 in adipose and uterine tissues. Furthermore, our study confirmed that adipocyte derived EV increased EC cell proliferation, migration and xenograft tumor growth. Additionally, we identified that the small molecule inhibitors (HO-3867) or Metformin inhibited EV secretion in vitro and in vivo, demonstrating significant inhibition of high glucose or adipocyte-mediated EC cell proliferation and a reduction in body weight and adipose tissue accumulation when administered to HFD mice. Moreover, HO-3867 or Metformin treatment inhibited HFD induced hyperplasia (precursor of EC) by altering the expression of EV-regulated proteins and decreasing oncogenic protein expression levels. This study provides critical insights into the mechanisms underpinning obesity-mediated EV secretion with oncogenic protein expression, shedding light on their role in EC pathogenesis. Additionally, it offers pre-clinical evidence supporting the initiation of novel studies for EV-targeted therapies aimed at preventing obesity-mediated EC.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests. Ethics approval and consent to participate: All procedures used in this study were authorized and conducted according to the guidelines of the Ohio State University Research Institute Ethics Committee. All animal experiments were following the Animal Experimentation Ethics of the Ohio State University Animal Experimentation Research Lab, and the ethics approval number for animal experimentation was 2012A00000008-R3. The use of stored human tissues in this study was approved by the Institutional Review Board of the Ohio State University Wexner Medical Center under Study Number: 2004C0124 and the Ohio State University’s OHRP Federal wide Assurance #00006378. No human subjects were directly consented for this study as the tissues were obtained from a biorepository.
Figures

References
-
- Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73:17–48. - PubMed
-
- Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371:569–78. - PubMed
-
- Renehan AG, MacKintosh ML, Crosbie EJ. Obesity and endometrial cancer: unanswered epidemiological questions. BJOG. 2016;123:175–8. - PubMed
-
- Hetemaki N, Mikkola TS, Tikkanen MJ, Wang F, Hamalainen E, Turpeinen U, et al. Adipose tissue estrogen production and metabolism in premenopausal women. J Steroid Biochem Mol Biol. 2021;209:105849. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

