Glucose-sensitive insulin with attenuation of hypoglycaemia
- PMID: 39415004
- PMCID: PMC11499270
- DOI: 10.1038/s41586-024-08042-3
Glucose-sensitive insulin with attenuation of hypoglycaemia
Abstract
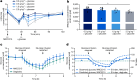
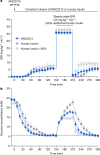
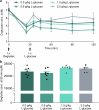
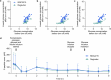
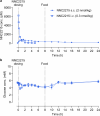
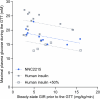
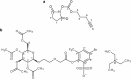
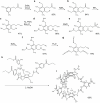
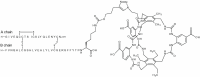
The risk of inducing hypoglycaemia (low blood glucose) constitutes the main challenge associated with insulin therapy for diabetes1,2. Insulin doses must be adjusted to ensure that blood glucose values are within the normal range, but matching insulin doses to fluctuating glucose levels is difficult because even a slightly higher insulin dose than needed can lead to a hypoglycaemic incidence, which can be anything from uncomfortable to life-threatening. It has therefore been a long-standing goal to engineer a glucose-sensitive insulin that can auto-adjust its bioactivity in a reversible manner according to ambient glucose levels to ultimately achieve better glycaemic control while lowering the risk of hypoglycaemia3. Here we report the design and properties of NNC2215, an insulin conjugate with bioactivity that is reversibly responsive to a glucose range relevant for diabetes, as demonstrated in vitro and in vivo. NNC2215 was engineered by conjugating a glucose-binding macrocycle4 and a glucoside to insulin, thereby introducing a switch that can open and close in response to glucose and thereby equilibrate insulin between active and less-active conformations. The insulin receptor affinity for NNC2215 increased 3.2-fold when the glucose concentration was increased from 3 to 20 mM. In animal studies, the glucose-sensitive bioactivity of NNC2215 was demonstrated to lead to protection against hypoglycaemia while partially covering glucose excursions.
© 2024. The Author(s).
Conflict of interest statement
T.H.-J., T.K., C.L.B., J.S., C.F., P.K.N., E.N., A.R.M., L.L., K.S.H., G.I., E.J., D.G., B.F.H., T.A.P., J.K., K.-M.P., H.H.F.R., L.A., J.J.F., A.V.N.-W., P.S. and R.S. are current or previous employees and shareholders of Novo Nordisk. A.P.D., R.A.T., M.T., M.G.O. and A.C. are listed as inventors on patent WO2018167503 (Davis, A. et al., macrocyclic compounds, 20 September 2018). T.H.-J., T.K., A.R.M., A.P.D., A.C. and P.S. are listed as inventors on patent WO2020058322 (Hoeg-Jensen, T. et al., glucose sensitive insulins and uses thereof, 26 March 2020). T.H.-J., T.K., A.R.M., L.L., K.S.H., A.P.D., M.T., A.C., P.S. and R.S. are listed as inventors on patent WO2023144240 (Hoeg-Jensen, T. et al., glucose sensitive insulin derivatives and uses thereof, 3 August 2023). The other authors declare no competing interests.
Figures





Comment in
-
Glucose-sensitive insulin protects against hypoglycaemia.Nat Rev Drug Discov. 2024 Dec;23(12):894. doi: 10.1038/d41573-024-00171-8. Nat Rev Drug Discov. 2024. PMID: 39468333 No abstract available.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

