Dopamine dynamics are dispensable for movement but promote reward responses
- PMID: 39415006
- PMCID: PMC11718420
- DOI: 10.1038/s41586-024-08038-z
Dopamine dynamics are dispensable for movement but promote reward responses
Abstract
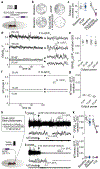
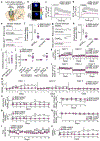
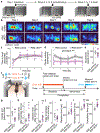
Dopamine signalling modes differ in kinetics and spatial patterns of receptor activation1,2. How these modes contribute to motor function, motivation and learning has long been debated3-21. Here we show that action-potential-induced dopamine release is dispensable for movement initiation but supports reward-oriented behaviour. We generated mice with dopamine-neuron-specific knockout of the release site organizer protein RIM to disrupt action-potential-induced dopamine release. In these mice, rapid in vivo dopamine dynamics were strongly impaired, but baseline dopamine persisted and fully supported spontaneous movement. Conversely, reserpine-mediated dopamine depletion or blockade of dopamine receptors disrupted movement initiation. The dopamine precursor L-DOPA reversed reserpine-induced bradykinesia without restoring fast dopamine dynamics, a result that substantiated the conclusion that these dynamics are dispensable for movement initiation. In contrast to spontaneous movement, reward-oriented behaviour was impaired in dopamine-neuron-specific RIM knockout mice. In conditioned place preference and two-odour discrimination tasks, the mice effectively learned to distinguish the cues, which indicates that reward-based learning persists after RIM ablation. However, the performance vigour was reduced. During probabilistic cue-reward association, dopamine dynamics and conditioned responses assessed through anticipatory licking were disrupted. These results demonstrate that action-potential-induced dopamine release is dispensable for motor function and subsecond precision of movement initiation but promotes motivation and performance during reward-guided behaviours.
© 2024. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
Figures












References
-
- da Silva JA, Tecuapetla F, Paixao V & Costa RM Dopamine neuron activity before action initiation gates and invigorates future movements. Nature 554, 244–248 (2018). - PubMed
References for Methods and Extended data
-
- Backman CM et al. Characterization of a mouse strain expressing Cre recombinase from the 3’ untranslated region of the dopamine transporter locus. Genesis 44, 383–390 (2006). - PubMed
-
- Allen Institute for Brain Science (2004). Allen Mouse Brain Atlas [mouse, P56, coronal]. atlas.brain-map.org (2011).
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

