Immunomodulatory effect of IFN-γ licensed adipose-mesenchymal stromal cells in an in vitro model of inflammation generated by SARS-CoV-2 antigens
- PMID: 39415027
- PMCID: PMC11484699
- DOI: 10.1038/s41598-024-75776-5
Immunomodulatory effect of IFN-γ licensed adipose-mesenchymal stromal cells in an in vitro model of inflammation generated by SARS-CoV-2 antigens
Abstract
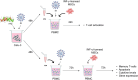
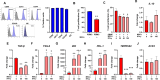
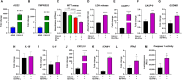
In recent years, clinical studies have shown positive results of the application of Mesenchymal Stromal Cells (MSCs) in severe cases of COVID-19. However, the mechanisms of immunomodulation of IFN-γ licensed MSCs in SARS-CoV-2 infection are only partially understood. In this study, we first tested the effect of IFN-γ licensing in the MSC immunomodulatory profile. Then, we established an in vitro model of inflammation by exposing Calu-3 lung cells to SARS-CoV-2 nucleocapsid and spike (NS) antigens, and determined the toxicity of SARS-CoV-2 NS antigen and/or IFN-γ stimulation to Calu-3. The conditioned medium (iCM) generated by Calu-3 cells exposed to IFN-γ and SARS-CoV-2 NS antigens was used to stimulate T-cells, which were then co-cultured with IFN-γ-licensed MSCs. The exposure to IFN-γ and SARS-CoV-2 NS antigens compromised the viability of Calu-3 cells and induced the expression of the inflammatory mediators ICAM-1, CXCL-10, and IFN-β by these cells. Importantly, despite initially stimulating T-cell activation, IFN-γ-licensed MSCs dramatically reduced IL-6 and IL-10 levels secreted by T-cells exposed to NS antigens and iCM. Moreover, IFN-γ-licensed MSCs were able to significantly inhibit T-cell apoptosis induced by SARS-CoV-2 NS antigens. Taken together, our data show that, in addition to reducing the level of critical cytokines in COVID-19, IFN-γ-licensed MSCs protect T-cells from SARS-CoV-2 antigen-induced apoptosis. Such observations suggest that MSCs may contribute to COVID-19 management by preventing the lymphopenia and immunodeficiency observed in critical cases of the disease.
Keywords: COVID-19; IFN-γ; Mesenchymal stem cells; Nucleocapsid; SARS-CoV-2; Spike; T-cells.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Figures



Similar articles
-
Inflammatory licensed equine MSCs are chondroprotective and exhibit enhanced immunomodulation in an inflammatory environment.Stem Cell Res Ther. 2018 Apr 3;9(1):82. doi: 10.1186/s13287-018-0840-2. Stem Cell Res Ther. 2018. PMID: 29615127 Free PMC article.
-
Immunomodulatory potential of cytokine-licensed human bone marrow-derived mesenchymal stromal cells correlates with potency marker expression profile.Stem Cells. 2024 Dec 6;42(12):1040-1054. doi: 10.1093/stmcls/sxae053. Stem Cells. 2024. PMID: 39208292 Free PMC article.
-
T-Cell Epitope Mapping of SARS-CoV-2 Reveals Coordinated IFN-γ Production and Clonal Expansion of T Cells Facilitates Recovery from COVID-19.Viruses. 2024 Jun 22;16(7):1006. doi: 10.3390/v16071006. Viruses. 2024. PMID: 39066169 Free PMC article.
-
Mesenchymal stem/stromal cells as a valuable source for the treatment of immune-mediated disorders.Stem Cell Res Ther. 2021 Mar 18;12(1):192. doi: 10.1186/s13287-021-02265-1. Stem Cell Res Ther. 2021. Retraction in: Stem Cell Res Ther. 2024 Apr 22;15(1):110. doi: 10.1186/s13287-024-03733-0. PMID: 33736695 Free PMC article. Retracted. Review.
-
Mesenchymal stem cells immunomodulation: The road to IFN-γ licensing and the path ahead.Cytokine Growth Factor Rev. 2019 Jun;47:32-42. doi: 10.1016/j.cytogfr.2019.05.006. Epub 2019 May 18. Cytokine Growth Factor Rev. 2019. PMID: 31129018 Review.
Cited by
-
The influence of asthmatic inflammation and house dust mite (HDM) exposure on abundance, immune-modulatory potential, and differentiation capacity of the lung-resident mesenchymal stem cells (lrMSCs).Stem Cell Res Ther. 2025 Jul 22;16(1):396. doi: 10.1186/s13287-025-04520-1. Stem Cell Res Ther. 2025. PMID: 40696474 Free PMC article.
-
Ethyl β-carboline-3-carboxylate targets PRDX5/c-Jun axis for novel therapeutic strategy against cervical cancer.Discov Oncol. 2025 Aug 7;16(1):1498. doi: 10.1007/s12672-025-03306-4. Discov Oncol. 2025. PMID: 40775543 Free PMC article.
References
-
- Pittenger, M. F. et al. Multilineage potential of adult human mesenchymal stem cells. Science. 284, 143–147 (1999). - PubMed
-
- Covas, D. T. et al. Multipotent mesenchymal stromal cells obtained from diverse human tissues share functional properties and gene-expression profile with CD146 + perivascular cells and fibroblasts. Exp. Hematol.36, 642–654 (2008). - PubMed
-
- Campagnoli, C. et al. Identification of mesenchymal stem/progenitor cells in human first-trimester fetal blood, liver, and bone marrow. Blood. 98, 2396–2402 (2001). - PubMed
-
- in ’t Anker, P. S. et al. Mesenchymal stem cells in human second-trimester bone marrow, liver, lung, and spleen exhibit a similar immunophenotype but a heterogeneous multilineage differentiation potential. Haematologica. 88, 845–852 (2003). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

