Radiomics approach for identifying radiation-induced normal tissue toxicity in the lung
- PMID: 39415029
- PMCID: PMC11484882
- DOI: 10.1038/s41598-024-75993-y
Radiomics approach for identifying radiation-induced normal tissue toxicity in the lung
Abstract
The rapidly evolving field of radiomics has shown that radiomic features are able to capture characteristics of both tumor and normal tissue that can be used to make accurate and clinically relevant predictions. In the present study we sought to determine if radiomic features can characterize the adverse effects caused by normal tissue injury as well as identify if human embryonic stem cell (hESC) derived extracellular vesicle (EV) treatment can resolve certain adverse complications. A cohort of 72 mice (n = 12 per treatment group) were exposed to X-ray radiation to the whole lung (3 × 8 Gy) or to the apex of the right lung (3 × 12 Gy), immediately followed by retro-orbital injection of EVs. Cone-Beam Computed Tomography images were acquired before and 2 weeks after treatment. In total, 851 radiomic features were extracted from the whole lungs and < 20 features were selected to train and validate a series of random forest classification models trained to predict radiation status, EV status and treatment group. It was found that all three classification models achieved significantly high prediction accuracies on a validation subset of the dataset (AUCs of 0.91, 0.86 and 0.80 respectively). In the locally irradiated lung, a significant difference between irradiated and unirradiated groups as well as an EV sparing effect were observed in several radiomic features that were not seen in the unirradiated lung (including wavelet-LLH Kurtosis, wavelet HLL Large Area High Gray Level Emphasis, and Gray Level Non-Uniformity). Additionally, a radiation difference was not observed in a secondary comparison cohort, but there was no impact of imaging machine parameters on the radiomic signature of unirradiated mice. Our data demonstrate that radiomics has the potential to identify radiation-induced lung injury and could be applied to predict therapeutic efficacy at early timepoints.
Keywords: Extracellular vesicles; Machine learning; Radiomics; Radiotherapy.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
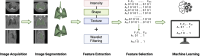
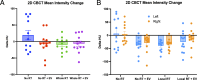
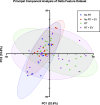

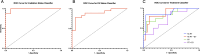


Update of
-
Radiomics approach for identifying radiation-induced normal tissue toxicity in the lung.Res Sq [Preprint]. 2024 Feb 23:rs.3.rs-3951996. doi: 10.21203/rs.3.rs-3951996/v1. Res Sq. 2024. Update in: Sci Rep. 2024 Oct 16;14(1):24256. doi: 10.1038/s41598-024-75993-y. PMID: 38464210 Free PMC article. Updated. Preprint.
References
-
- Gardin, I. et al. Radiomics: principles and radiotherapy applications. Crit. Rev. Oncol. Hematol. 138, 44–50 (2019). - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

