Ex vivo imaging reveals the spatiotemporal control of ovulation
- PMID: 39415041
- PMCID: PMC11567896
- DOI: 10.1038/s41556-024-01524-6
Ex vivo imaging reveals the spatiotemporal control of ovulation
Abstract
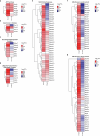
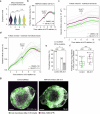
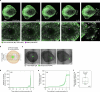
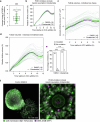
During ovulation, an egg is released from an ovarian follicle, ready for fertilization. Ovulation occurs inside the body, impeding direct studies of its progression. Therefore, the exact mechanisms that control ovulation have remained unclear. Here we devised live imaging methods to study the entire process of ovulation in isolated mouse ovarian follicles. We show that ovulation proceeds through three distinct phases, follicle expansion (I), contraction (II) and rupture (III), culminating in the release of the egg. Follicle expansion is driven by hyaluronic acid secretion and an osmotic gradient-directed fluid influx into the follicle. Then, smooth muscle cells in the outer follicle drive follicle contraction. Follicle rupture begins with stigma formation, followed by the exit of follicular fluid and cumulus cells and the rapid release of the egg. These results establish a mechanistic framework for ovulation, a process of fundamental importance for reproduction.
© 2024. The Author(s).
Conflict of interest statement
Figures







References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

