Lung and liver editing by lipid nanoparticle delivery of a stable CRISPR-Cas9 ribonucleoprotein
- PMID: 39415058
- PMCID: PMC12000389
- DOI: 10.1038/s41587-024-02437-3
Lung and liver editing by lipid nanoparticle delivery of a stable CRISPR-Cas9 ribonucleoprotein
Abstract
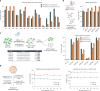
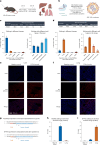
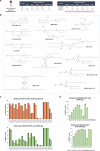
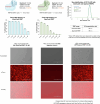
Lipid nanoparticle (LNP) delivery of clustered regularly interspaced short palindromic repeat (CRISPR) ribonucleoproteins (RNPs) could enable high-efficiency, low-toxicity and scalable in vivo genome editing if efficacious RNP-LNP complexes can be reliably produced. Here we engineer a thermostable Cas9 from Geobacillus stearothermophilus (GeoCas9) to generate iGeoCas9 variants capable of >100× more genome editing of cells and organs compared with the native GeoCas9 enzyme. Furthermore, iGeoCas9 RNP-LNP complexes edit a variety of cell types and induce homology-directed repair in cells receiving codelivered single-stranded DNA templates. Using tissue-selective LNP formulations, we observe genome-editing levels of 16‒37% in the liver and lungs of reporter mice that receive single intravenous injections of iGeoCas9 RNP-LNPs. In addition, iGeoCas9 RNPs complexed to biodegradable LNPs edit the disease-causing SFTPC gene in lung tissue with 19% average efficiency, representing a major improvement over genome-editing levels observed previously using viral or nonviral delivery strategies. These results show that thermostable Cas9 RNP-LNP complexes can expand the therapeutic potential of genome editing.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: The Regents of the University of California have patents issued and pending for CRISPR technologies on which K.C., H.H., S.Z., N.M. and J.A.D. are inventors. J.A.D. is a cofounder of Azalea Therapeutics, Caribou Biosciences, Editas Medicine, Evercrisp, Scribe Therapeutics, Intellia Therapeutics and Mammoth Biosciences. J.A.D. is a scientific advisory board member at Evercrisp, Caribou Biosciences, Intellia Therapeutics, Scribe Therapeutics, Mammoth Biosciences, The Column Group and Inari. J.A.D. is also an advisor for Aditum Bio. J.A.D. is chief science advisor to Sixth Street, is a director at Altos, Johnson & Johnson and Tempus and has research projects sponsored by Apple Tree Partners. N.M. and H.H. are founders of Opus Biosciences. The other authors declare no competing interests.
Figures











Update of
-
Lung and liver editing by lipid nanoparticle delivery of a stable CRISPR-Cas9 RNP.bioRxiv [Preprint]. 2023 Nov 15:2023.11.15.566339. doi: 10.1101/2023.11.15.566339. bioRxiv. 2023. Update in: Nat Biotechnol. 2025 Sep;43(9):1445-1457. doi: 10.1038/s41587-024-02437-3. PMID: 38014175 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Grants and funding
- RM1HG009490/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- UM1 AI164559/AI/NIAID NIH HHS/United States
- R21 HL173710/HL/NHLBI NIH HHS/United States
- RM1 HG009490/HG/NHGRI NIH HHS/United States
- U19 AI135990/AI/NIAID NIH HHS/United States
- UH3 NS115599/NS/NINDS NIH HHS/United States
- U19 NS132303/NS/NINDS NIH HHS/United States
- R01MH125979-01/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- R01 MH125979/MH/NIMH NIH HHS/United States
- U19 AI171110/AI/NIAID NIH HHS/United States
- DISC2-14097/California Institute for Regenerative Medicine (CIRM)
- DISC2-14045/California Institute for Regenerative Medicine (CIRM)
- U54 AI170792/AI/NIAID NIH HHS/United States
- UG3 NS115599/NS/NINDS NIH HHS/United States
- UM1AI164559/U.S. Department of Health & Human Services | NIH | National Institute of Allergy and Infectious Diseases (NIAID)
- UH3 AI150552/AI/NIAID NIH HHS/United States
- U01 AI142817/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

