The impact of physical activity on progression-free and overall survival in metastatic breast cancer based on molecular subtype
- PMID: 39415149
- PMCID: PMC11481816
- DOI: 10.1186/s12885-024-13038-3
The impact of physical activity on progression-free and overall survival in metastatic breast cancer based on molecular subtype
Abstract
Background: Although adequate physical activity has been shown to be beneficial in early breast cancer, evidence in metastatic breast cancer is sparse and contradictory, which could be related to distinct effects of physical activity on the different molecular cancer subtypes. Therefore, we here evaluated the effect of physical activity on progression-free and overall survival (PFS, OS) in metastatic breast cancer, specifically looking at molecular subtypes.
Methods: International Physical Activity Questionnaire (IPAQ) questionnaires, filled out by patients enrolled in the prospective PRAEGNANT registry (NCT02338167; n = 1,270) were used to calculate metabolic equivalent task (MET) minutes, which were subsequently categorized into low (n = 138), moderate (n = 995) or high IPAQ categories (n = 137). Cox regression analyses were used to evaluate the impact of IPAQ categories and its interaction with molecular subtypes on PFS and OS.
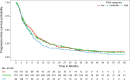
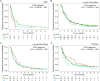
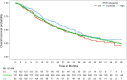
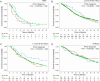
Results: Patient and tumor characteristics were equally distributed across IPAQ categories. HER2pos, HRpos and TNBC were present in 23.1%, 65.7% and 11.2% of patients, respectively. IPAQ scores did not have an impact on PFS and OS in addition to established prognostic factors, either overall or in particular molecular subtypes (PFS: p = 0.33 and OS: p = 0.08, likelihood ratio test). Exploratory analyses showed higher overall survival rates for high IPAQ categories compared to low/moderate IPAQ categories in luminal B-like breast cancer.
Conclusions: Self-reported physical activity using the IPAQ questionnaire did not significantly affect PFS or OS in patients suffering from metastatic breast cancer. Nevertheless, some hypothesis-generating differences between molecular subtypes could be observed, which may be interesting to evaluate further.
Keywords: IPAQ; Metastatic breast cancer; Molecular subtype; Physical activity.
© 2024. The Author(s).
Conflict of interest statement
ADH: has received honoraria from Roche, Novartis, Lilly, MSD, AstraZeneca, Seagen, GSK, Exact Science, Riemser, Teva, Onkowissen, Gilead, Stemline Therapeutics, Pfizer, Amgen, Pierre Fabre and Eisai and travel support from Roche, Novartis, Lilly, AstraZeneca, GSK, Exact Science, Gilead, Stemline Therapeutics and Pfizer. MW: received speaker honoraria from AstraZeneca, Celgene, Roche, MSD and Novartis. H-CK: has received honoraria from Pfizer, Novartis, Roche, Genomic Health/Exact Sciences, Amgen, AstraZeneca, Riemser, Carl Zeiss Meditec, TEVA, Theraclion, Janssen-Cilag, GSK, LIV Pharma, Lily, SurgVision, Onkowissen, Gilead, Daiichi Sankyo and MSD, travel support from Carl Zeiss Meditec, LIV Pharma, Novartis, Amgen, Pfizer, Daiichi Sankyo, Tesaro, Gilead and Stemline Therapeutics and owns stock of Theraclion SA. PH: has received honoraria from Amgen, Novartis, Hexal and Pfizer. HT: has received honoraria from Novartis, Roche, Celgene, Teva, and Pfizer, and travel support from Roche, Celgene and Pfizer. JE: has received honoraria/travel support from Roche, Celgene, Novartis, Pfizer, Lilly, Pierre Fabre, Teva, and Tesaro, AstraZeneca, Daiichi Sankyo, Seagen, Gilead, Stemline Therapeutics, ClinSol. DL: has received honoraria from Amgen, Loreal, Pfizer, Novartis, Eli Lilly, Samsung, Celgene, Astra Zeneca, Teva and GSK. VM: has received speaker honoraria from AstraZeneca, Daiichi Sankyo, Eisai, Pfizer, MSD, Medac, Novartis, Roche, Seagen, Onkowissen, high5 Oncology, Medscape, Gilead, Pierre Fabre, iMED Institut. Has received consultancy honoraria from Roche, Pierre Fabre, PINK, ClinSol, Novartis, MSD, Daiichi Sankyo, Eisai, Lilly, Seagen, Gilead, Stemline Therapeutics. Has received institutional research support from Novartis, Roche, Seagen, Genentech, AstraZeneca. Has received travel grants from AstraZeneca, Roche, Pfizer, Daiichi Sankyo, Gilead. LLM: received honoraria from Amgen, AstraZeneca, Celgene, Gilead, Lilly, MSD, Novartis, Pfizer, Roche and Eisai for advisory boards, lectures and travel support. EB: has received honoraria from Novartis, Celgene, Eisai, Daiichi Sankyo, Merrimack, AstraZeneca, Riemser, Pfizer, Hexal, Amgen, and onkowissen.de for consulting, clinical research management, or medical education activities. PW: has received honoraria from Roche, Novartis, Amgen, AstraZeneca, Pfizer, MSD, Clovis, Tesaro, Celgene, Teva, Eisai, Daiichi Sankyo, Seagen and Eli Lilly. CH: has received honoraria from Amgen, Celgene, Oncovis, Roche, and Pfizer. HH: received speaker honoraria from: Novartis Pharma GmbH and LEO Pharma GmbH and grant/research support from: Novartis Pharma GmbH. CCH: received honoraria from AstraZeneca, Daiichi Sankyo, Eisai, Novartis, Pfizer, Roche, Gilead and MSD as well as support for attending meetings from Daiichi Sankyo. CK: has received honoraria from Amgen, Roche, Teva, Novartis, MSD, Axios, and Riemser. PAF: has received honoraria from Roche, Pfizer, Novartis, and Celgene; his institution conducts research for Novartis. RW: has received honoraria from Agendia, Amgen, APOGHEVA, Aristo, AstraZeneca, Celgene, Clovis Oncology, Daiichi Sankyo, Eisai, Esteve, Exact Sciences, Gilead, GSK, Hexal, Lilly, Medstrom Medical, MSD, Mundipharma, Mylan, Nanostring, Novartis, Odonate, Paxman, Palleos, Pfizer, Pierre Fabre, PINK, Puma Biotechnolgogy, Riemser, Roche, Sandoz/Hexal, Sanofi Genzyme, Seattle Genetics/Seagen, Sidekick, Stemline Therapeutics, Tesaro Bio, Teva, Veracyte, Viatris, Wiley, FOMF, Aurikamed, ClinSol, Pomme Med, medconcept, MCI, MediSeminar. MU: has received honoraria for advisory boards and travel support, payed to the employer from Abbvie, Amgen, AstraZeneca, BMS, Celgene, Daiichi Sankyo, Eisai, Lilly Deutschland, Lilly Int., MSD, Mundipharma, Myriad Genetics, Odonate, Pfizer, Puma Biotechnology, Roche, Sanofi Aventis Deutschland, Teva Pharmaceuticals Ind Ltd, Novartis, Pierre Fabre, Clovis Oncology, and Seattle Genetics. WJ: as received honoraria and research grants from Sanofi-Aventis, Novartis, Lilly, Pfizer, Roche, Chugai, AstraZeneca, MSD, and Daiichi Sankyo. F-AT: has received speaker and consultancy honoraria from AstraZeneca, Gilead, GSK, MSD, Novartis, Onkowissen, Pfizer, Roche. MPL: has received honoraria from Lilly, Pfizer, Roche, MSD, Hexal, Novartis, AstraZeneca, Eisai, Exact Sciences, Agendia, Daiichi Sankyo, Grünenthal, Gilead, Pierre Fabre, PharmaMar, Samantree, Endomag, and medac for advisory boards, lectures, and travel support. SYB: received honoraria from Roche, Novartis, Pfizer, MSD, Teva, AstraZeneca. TNF: received honoraria from Novartis, Roche, Pfizer, TEVA, Daiichi Sankyo, AstraZeneca and MSD. AS: has received honoraria from Roche, Celgene, AstraZeneca, Novartis, Pfizer, Zuckschwerdt Verlag GmbH, Georg Thieme Verlag, Aurikamed GmbH, MCI Deutschland GmbH, bsh medical communications GmbH, and promedicis GmbH. All remaining authors have nothing to declare.
Figures

References
-
- Krebsregisterdaten, Z.f. and G.d.e.K.i.D. e.V., Cancer in Germany [Krebs in Deutschland]. Robert Koch Institut, 2019. 14.
-
- Gobbini E, et al. Time trends of overall survival among metastatic breast cancer patients in the real-life ESME cohort. Eur J Cancer. 2018;96:17–24. - PubMed
-
- Juvet LK, et al. The effect of exercise on fatigue and physical functioning in breast cancer patients during and after treatment and at 6 months follow-up: A meta-analysis. Breast. 2017;33:166–77. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

