Tumor-intrinsic role of ICAM-1 in driving metastatic progression of triple-negative breast cancer through direct interaction with EGFR
- PMID: 39415210
- PMCID: PMC11481280
- DOI: 10.1186/s12943-024-02150-4
Tumor-intrinsic role of ICAM-1 in driving metastatic progression of triple-negative breast cancer through direct interaction with EGFR
Abstract
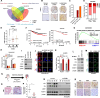
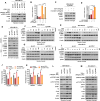
Triple-negative breast cancer (TNBC), the most aggressive subtype, presents a critical challenge due to the absence of approved targeted therapies. Hence, there is an urgent need to identify effective therapeutic targets for this condition. While epidermal growth factor receptor (EGFR) is prominently expressed in TNBC and recognized as a therapeutic target, anti-EGFR therapies have yet to gain approval for breast cancer treatment due to their associated side effects and limited efficacy. Here, we discovered that intercellular adhesion molecule-1 (ICAM-1) exhibits elevated expression levels in metastatic breast cancer and serves as a pivotal binding adaptor for EGFR activation, playing a crucial role in malignant progression. The activation of EGFR by tumor-expressed ICAM-1 initiates biased signaling within the JAK1/STAT3 pathway, consequently driving epithelial-to-mesenchymal transition and facilitating heightened metastasis without influencing tumor growth. Remarkably, ICAM-1-neutralizing antibody treatment significantly suppressed cancer metastasis in a breast cancer orthotopic xenograft mouse model. In conclusion, our identification of ICAM-1 as a novel tumor intrinsic regulator of EGFR activation offers valuable insights for the development of TNBC-specific anti-EGFR therapies.
Keywords: EGFR; ICAM-1; JAK1/STAT3 signaling; Targeted therapy; Triple-negative breast cancer.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Scully OJ, Bay BH, Yip G, Yu Y. Breast cancer metastasis. Cancer Genomics Proteomics. 2012;9:311–20. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

