The efficacy of wrapping with polyglycolic acid mesh and fibrin glue in preventing clinically relevant pancreatic fistula after minimally invasive distal pancreatectomy (WRAP Study): study protocol for a multicenter randomized controlled trial in Japan
- PMID: 39415231
- PMCID: PMC11481437
- DOI: 10.1186/s12893-024-02610-0
The efficacy of wrapping with polyglycolic acid mesh and fibrin glue in preventing clinically relevant pancreatic fistula after minimally invasive distal pancreatectomy (WRAP Study): study protocol for a multicenter randomized controlled trial in Japan
Abstract
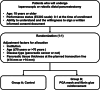
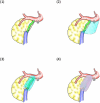
Background: Postoperative pancreatic fistula (POPF) continues to be the most common complication after distal pancreatectomy (DP). Recent advancements in surgical techniques have established minimally invasive distal pancreatectomy (MIDP) as the standard treatment for various conditions, including pancreatic cancer. However, MIDP has not demonstrated a clear advantage over open DP in terms of POPF rates, indicating the need for additional strategies to prevent POPF in MIDP. This trial (WRAP study) aims to evaluate the efficacy of wrapping the pancreatic stump with polyglycolic acid (PGA) mesh and fibrin glue in preventing clinically relevant (CR-) POPF following MIDP.
Methods: This multicenter, randomized controlled trial will include patients scheduled for laparoscopic or robotic DP for tumors in the pancreatic body and/or tail. Eligible participants will be centrally randomized into either the control group (Group A) or the intervention group (Group B), where the pancreatic stump will be reinforced by PGA mesh and fibrin glue. In both groups, pancreatic transection will be performed using a bioabsorbable reinforcement-attached stapler. A total of 172 patients will be enrolled across 14 high-volume centers in Japan. The primary endpoint is the incidence of CR-POPF (International Study Group of Pancreatic Surgery grade B/C).
Discussion: The WRAP study will determine whether the reinforcement of the pancreatic stump with PGA mesh and fibrin glue, a technique whose utility has been previously debated, could become the best practice in the era of MIDP, thereby enhancing its safety.
Trial registration: This trial was registered with the Japan Registry of Clinical Trials on June 15, 2024 (jRCTs032240120).
Keywords: Distal pancreatectomy; Fibrin glue; Minimally invasive surgery; Pancreatic fistula; Polyglycolic acid mesh.
© 2024. The Author(s).
Conflict of interest statement
Atsushi Oba has been awarded a grant (Bayer Yakuhin, Ltd.) to conduct an observational study to investigate the clinical impact of EOB-MRI. The other authors have no conflicts of interest to declare. Sohei Satoi declared research fundings from Nihon Servier, Amino-up co. and Boston Scientific.
Figures
References
-
- Knaebel H, Diener M, Wente M, Büchler M, Seiler C. Systematic review and meta-analysis of techniques for closure of the pancreatic remnant after distal pancreatectomy. Br J Surg. 2005;92:539–46. - PubMed
-
- Strasberg SM, Linehan DC, Clavien PA, Barkun JS. Proposal for definition and severity grading of pancreatic anastomosis failure and pancreatic occlusion failure. Surgery. 2007;141:420–6. - PubMed
-
- Butturini G, Marcucci S, Molinari E, Mascetta G, Landoni L, Crippa S, Bassi C. Complications after pancreaticoduodenectomy: the problem of current definitions. J Hepatobiliary Pancreat Surg. 2006;13:207–11. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical