N-acetylcysteine supplementation improves endocrine-metabolism profiles and ovulation induction efficacy in polycystic ovary syndrome
- PMID: 39415242
- PMCID: PMC11484282
- DOI: 10.1186/s13048-024-01528-8
N-acetylcysteine supplementation improves endocrine-metabolism profiles and ovulation induction efficacy in polycystic ovary syndrome
Abstract
Background: Polycystic ovary syndrome (PCOS) affects 6-20% of women worldwide, with insulin resistance and hyperinsulinemia occurring in 50-70% of patients. Hyperinsulinemia exacerbates oxidative stress, contributing to PCOS pathogenesis. N-acetylcysteine (NAC) is an antioxidant and insulin sensitizer that shows promise as a therapeutic for PCOS. Our current study aimed to investigate the effects of NAC supplementation on endocrine-metabolic parameters in PCOS mice and its effect on ovulation induction (OI) efficacy in women with PCOS.
Methods: Female C57BL/6 mice were orally administered letrozole (LE) to induce PCOS and then randomly divided into groups receiving daily oral administration of 160 mg/kg NAC (PCOS + NAC group), 200 mg/kg metformin (PCOS + Met group), or 0.5% carboxymethyl cellulose (drug solvent) (pure PCOS group) for 12 days. Healthy female mice served as pure controls. Estrous cycles were monitored during the intervention. Metabolic and hormone levels, ovarian phenotypes, antioxidant activity in ovarian tissues, and oxidative stress levels in oocytes were assessed post-intervention. Furthermore, a pragmatic, randomized, controlled clinical study was conducted with 230 PCOS women, randomly assigned to the NAC group (1.8 g/day oral NAC, n = 115) or the control group (n = 115). Patients in both groups underwent ≤ 3 cycles of OI with sequential LE and urinary follicle-stimulating hormone (uFSH). Cycle characteristics and pregnancy outcomes were compared between groups.
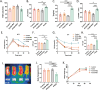
Results: Similar to metformin, NAC supplementation significantly improved the estrous cycles and ovarian phenotypes of PCOS mice; reduced the LH concentration, LH/FSH ratio, and T level; and increased glucose clearance and insulin sensitivity. Notably, NAC significantly reduced oocyte ROS levels and increased the mitochondrial membrane potential in PCOS mice. Additionally, NAC significantly enhanced enzymatic and nonenzymatic antioxidant activities in PCOS mouse ovaries, whereas metformin had no such effect. In the clinical trial, compared to women in the control group, women receiving NAC had significantly lower average uFSH dosage and duration (p < 0.005) and significantly greater clinical pregnancy rates per OI cycle and cumulative clinical pregnancy rates per patient (p < 0.005).
Conclusion: NAC supplementation improved endocrine-metabolic parameters in PCOS mice and significantly enhanced OI efficacy with sequential LE and uFSH in women with PCOS. Therefore, NAC could be a valuable adjuvant in OI for women with PCOS.
Keywords: Insulin resistance; Metformin; N-acetylcysteine; Ovulation induction; Oxidative stress; Polycystic ovary syndrome; Pregnancy rate.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Shrivastava S, Conigliaro RL. Polycystic ovarian syndrome. Med Clin North Am. 2023;107(2):227–34. - PubMed
-
- Wu Q, et al. The prevalence of polycystic ovarian syndrome in Chinese women: a meta-analysis. Ann Palliat Med. 2021;10(1):74–87. - PubMed
-
- Sengupta P, Dutta S, Hassan MF. Polycystic ovary syndrome (PCOS) and oxidative stress. J Integr Sci Technol. 2024;12(3):752.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

