Targeting cancer-associated fibroblasts/tumor cells cross-talk inhibits intrahepatic cholangiocarcinoma progression via cell-cycle arrest
- PMID: 39415286
- PMCID: PMC11484308
- DOI: 10.1186/s13046-024-03210-9
Targeting cancer-associated fibroblasts/tumor cells cross-talk inhibits intrahepatic cholangiocarcinoma progression via cell-cycle arrest
Abstract
Background: Cancer-associated fibroblasts (CAFs), mainly responsible for the desmoplastic reaction hallmark of intrahepatic Cholangiocarcinoma (iCCA), likely have a role in tumor aggressiveness and resistance to therapy, although the molecular mechanisms involved are unknown. Aim of the study is to investigate how targeting hCAF/iCCA cross-talk with a Notch1 inhibitor, namely Crenigacestat, may affect cancer progression.
Methods: We used different in vitro models in 2D and established new 3D hetero-spheroids with iCCA cells and human (h)CAFs. The results were confirmed in a xenograft model, and explanted tumoral tissues underwent transcriptomic and bioinformatic analysis.
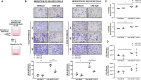
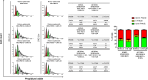
Results: hCAFs/iCCA cross-talk sustains increased migration of both KKU-M213 and KKU-M156 cells, while Crenigacestat significantly inhibits only the cross-talk stimulated migration. Hetero-spheroids grew larger than homo-spheroids, formed by only iCCA cells. Crenigacestat significantly reduced the invasion and growth of hetero- but not of homo-spheroids. In xenograft models, hCAFs/KKU-M213 tumors grew significantly larger than KKU-M213 tumors, but were significantly reduced in volume by Crenigacestat treatment, which also significantly decreased the fibrotic reaction. Ingenuity pathway analysis revealed that genes of hCAFs/KKU-M213 but not of KKU-M213 tumors increased tumor lesions, and that Crenigacestat treatment inhibited the modulated canonical pathways. Cell cycle checkpoints were the most notably modulated pathway and Crenigacestat reduced CCNE2 gene expression, consequently inducing cell cycle arrest. In hetero-spheroids, the number of cells increased in the G2/M cell cycle phase, while Crenigacestat significantly decreased cell numbers in the G2/M phase in hetero but not in homo-spheroids.
Conclusions: The hCAFs/iCCA cross-talk is a new target for reducing cancer progression with drugs such as Crenigacestat.
Keywords: Cholangiocarcinoma progression; Gamma secretase inhibitor; Liver cancer; Tumor microenvironment; Tumor stroma.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures






References
-
- Endo I, Gonen M, Yopp AC, Dalal KM, Zhou Q, Klimstra D, et al. Intrahepatic cholangiocarcinoma: rising frequency, improved survival, and determinants of outcome after resection. Ann Surg. 2008;248:84–96. - PubMed
-
- Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, et al. Cisplatin plus Gemcitabine versus Gemcitabine for biliary Tract Cancer. N Engl J Med. 2010;362:1273–81. - PubMed
-
- Moris D, Palta M, Kim C, Allen PJ, Morse MA, Lidsky ME. Advances in the treatment of intrahepatic cholangiocarcinoma: an overview of the current and future therapeutic landscape for clinicians. CA Cancer J Clin. 2023;73:198–222. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

