Evaluation of an oral small-molecule glucagon-like peptide-1 receptor agonist, lotiglipron, for type 2 diabetes and obesity: A dose-ranging, phase 2, randomized, placebo-controlled study
- PMID: 39415344
- PMCID: PMC11618248
- DOI: 10.1111/dom.16005
Evaluation of an oral small-molecule glucagon-like peptide-1 receptor agonist, lotiglipron, for type 2 diabetes and obesity: A dose-ranging, phase 2, randomized, placebo-controlled study
Abstract
Aim: The aim was to investigate the effects of lotiglipron, a once-daily, oral small-molecule glucagon-like peptide-1 (GLP-1) receptor agonist, in participants with type 2 diabetes (T2D) or obesity.
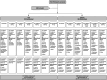
Materials and methods: A phase 2, randomized, double-blind, placebo-controlled, dose-ranging study investigated the efficacy and safety of lotiglipron. The study was terminated early for safety reasons after routine data and monitoring review. The planned analyses for the end points were modified prior to unblinding the study.
Results: In total, 901 participants were treated with at least one dose of the study drug (T2D cohort: n = 512, obesity cohort: n = 389). Although the majority of participants who were randomly assigned to higher doses did not reach their target maintenance dose, statistically significant changes in HbA1c and body weight were observed. In the T2D cohort, reductions in HbA1c were observed across all lotiglipron doses at week 16 (p < 0.0001), with least squares mean decreases up to -1.44% (90% confidence interval [CI]: -1.63, -1.26) (lotiglipron 80 mg), versus placebo, -0.07% (90% CI: -0.25, 0.11). In the obesity cohort, decreases in body weight were observed across all lotiglipron doses at week 20 (p < 0.01), up to -7.47% (90% CI: -8.50, -6.43) (lotiglipron 200 mg, five-step titration), versus placebo, -1.84% (90% CI: -2.85, -0.83). Across cohorts, the most frequently reported treatment-emergent adverse events were gastrointestinal related (most mild to moderate severity), with nausea being the most common (ranging from 4% [placebo] to 28.8% [80 mg] in the T2D cohort and 12.5% [placebo] to 60.6% [200 mg, four-step titration] in the obesity cohort). Transaminase elevations were observed in a subset of participants (6.6% and 6.0% of participants on lotiglipron in the T2D and obesity cohorts, respectively, compared with 1.6% on placebo in the obesity cohort).
Conclusions: The efficacy (HbA1c and/or body weight) of a range of lotiglipron doses was demonstrated in T2D and obesity cohorts. The safety profile was largely consistent with what has been previously known about the mechanism of action. Our results are unique in reporting elevations in liver transaminases in a subset of participants treated with lotiglipron, with attempts to identify the at-risk population unsuccessful and therefore clinical development of lotiglipron terminated.
Clinicaltrials: GOV: NCT05579977.
Keywords: HbA1c; PF‐07081532; diabetes; glucagon‐like peptide‐1 receptor agonist; lotiglipron; obesity; phase 2 study; weight loss.
© 2024 Pfizer,Inc. Diabetes, Obesity and Metabolism published by John Wiley & Sons Ltd.
Conflict of interest statement
Neeta B. Amin, Gina Buckley, Robert Frederich, Alexandra Palmer, Tilman Schuster, Nikolaos Tsamandouras and Qi Zhu are full‐time employees of Pfizer and may own shares/stock options in Pfizer. Sarah J. DuBrava and Margot Johnson were full‐time employees of Pfizer at the time of this study and may own shares/stock options in Pfizer. Amina Z. Haggag was a primary investigator and owns Pfizer stock. Szilard Vasas was a primary investigator of this study and others sponsored by Novo Nordisk, IONIS Pharma, Arrowhead Pharmaceuticals and New Amsterdam. Timothy R. Smith was a primary investigator of this study and others sponsored by Lilly, Amgen, Novo Nordisk, Novartis, Boehringer Ingelheim, AbbVie, Reata and Vertex. Witold Zmuda was a primary investigator and reports no other conflicts of interest.
Figures


References
-
- Engelmann J, Manuwald U, Rubach C, et al. Determinants of mortality in patients with type 2 diabetes: a review. Rev Endocr Metab Disord. 2016;17:129‐137. - PubMed
-
- Eeg‐Olofsson K, Cederholm J, Nilsson PM, et al. Risk of cardiovascular disease and mortality in overweight and obese patients with type 2 diabetes: an observational study in 13,087 patients. Diabetologia. 2009;52:65‐73. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

