Potential pathogenic roles of ferroptosis and cuproptosis in cadmium-induced or exacerbated cardiovascular complications in individuals with diabetes
- PMID: 39415790
- PMCID: PMC11479913
- DOI: 10.3389/fendo.2024.1461171
Potential pathogenic roles of ferroptosis and cuproptosis in cadmium-induced or exacerbated cardiovascular complications in individuals with diabetes
Abstract
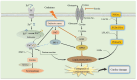
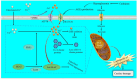
Diabetes and its complications are major diseases that affect human health. Diabetic cardiovascular complications such as cardiovascular diseases (CVDs) are the major complications of diabetes, which are associated with the loss of cardiovascular cells. Pathogenically the role of ferroptosis, an iron-dependent cell death, and cuproptosis, a copper-dependent cell death has recently been receiving attention for the pathogenesis of diabetes and its cardiovascular complications. How exposure to environmental metals affects these two metal-dependent cell deaths in cardiovascular pathogenesis under diabetic and nondiabetic conditions remains largely unknown. As an omnipresent environmental metal, cadmium exposure can cause oxidative stress in the diabetic cardiomyocytes, leading to iron accumulation, glutathione depletion, lipid peroxidation, and finally exacerbate ferroptosis and disrupt the cardiac. Moreover, cadmium-induced hyperglycemia can enhance the circulation of advanced glycation end products (AGEs). Excessive AGEs in diabetes promote the upregulation of copper importer solute carrier family 31 member 1 through activating transcription factor 3/transcription factor PU.1, thereby increasing intracellular Cu+ accumulation in cardiomyocytes and disturbing Cu+ homeostasis, leading to a decline of Fe-S cluster protein and reactive oxygen species accumulation in cardiomyocytes mitochondria. In this review, we summarize the available evidence and the most recent advances exploring the underlying mechanisms of ferroptosis and cuproptosis in CVDs and diabetic cardiovascular complications, to provide critical perspectives on the potential pathogenic roles of ferroptosis and cuproptosis in cadmium-induced or exacerbated cardiovascular complications in diabetic individuals.
Keywords: cadmium; cardiovascular diseases; cuproptosis; diabetes; diabetic cardiomyopathy; ferroptosis.
Copyright © 2024 Saedi, Tan, Watson, Wintergerst and Cai.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

