Vitamin B12 protects necrosis of acinar cells in pancreatic tissues with acute pancreatitis
- PMID: 39415850
- PMCID: PMC11480517
- DOI: 10.1002/mco2.686
Vitamin B12 protects necrosis of acinar cells in pancreatic tissues with acute pancreatitis
Abstract
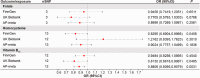
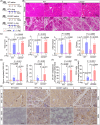
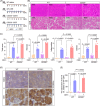
Pharmacological agents regarding the most optimal treatments of acute pancreatitis remain. One-carbon metabolism nutrients as therapeutic agents in many diseases might be involved in acute pancreatitis. The roles are acquired exploration in acute pancreatitis. We utilized Mendelian randomization to assess the causal impact of folate, homocysteine, and vitamin B12 (VB12) on acute pancreatitis. Wild-type and corresponding genetically modified mouse models were used to verify the genetic correlating findings. A negative association between genetically predicted serum VB12 levels and risks of acute pancreatitis was identified in human population. The transcobalamin receptor (TCblR)/CD320 gene ablation that decreased cellular VB12 uptake and ATP production in pancreatic tissues promoted necrosis, resulting in much severe pathological changes of induced acute pancreatitis in mice. VB12 pretreatment and posttreatment dramatically increased ATP levels in pancreatic tissues and reduced the necrosis, then the elevated levels of amylase in serum, the levels of CK-19, the activity of trypsin, and T lymphocyte infiltration in pancreatic tissues, prevented the pancreatic gross loss and ameliorated histopathological changes of mouse pancreases with induced acute pancreatitis. The results reveal that VB12 is potential as a therapeutic agent to inhibit tissue injuries and adaptive inflammatory responses in the pancreas in patients with acute pancreatitis.
Keywords: CD320‐ablation mouse; Mendelian randomization (MR); acute pancreatitis; vitamin B12.
© 2024 The Author(s). MedComm published by Sichuan International Medical Exchange & Promotion Association (SCIMEA) and John Wiley & Sons Australia, Ltd.
Conflict of interest statement
The authors declare they have no conflicts of interest.
Figures




References
-
- Boxhoorn L, Voermans RP, Bouwense SA, et al. Acute pancreatitis. Lancet. 2020;396(10252):726‐734. - PubMed
-
- Chua TY, Walsh RM, Baker ME, Stevens T. Necrotizing pancreatitis: diagnose. Clin J Med. 2017;84(8):639‐648. - PubMed
-
- Hines OJ, Pandol SJ. Management of severe acute pancreatitis. BMJ. 2019;367:l6227. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
