Selinexor in combination with dexamethasone with or without bortezomib in heavily pretreated multiple myeloma: A case series
- PMID: 39415919
- PMCID: PMC11474306
- DOI: 10.1002/jha2.913
Selinexor in combination with dexamethasone with or without bortezomib in heavily pretreated multiple myeloma: A case series
Abstract
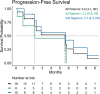
This report describes the characteristics and outcomes of 18 heavily pretreated patients with multiple myeloma (MM) who were subsequently treated with selinexor. This is a case series of 18 patients with MM who were treated with selinexor and dexamethasone (Sd) or selinexor, bortezomib, and dexamethasone (SVd) in 12 hospitals in the UK between 2019 and 2021. Eight patients received Sd and 10 patients received SVd. Patients received a median of five prior treatment lines, including immunomodulatory agents in 94% and proteasome inhibitors in 94%. Ten patients (55%) had triple-class refractory disease. Six of the 12 evaluable patients achieved ≥partial response. The median progression-free survival was 5.6 months, which was higher with SVd (5.7 months) than with Sd (2.1 months). The results support a treatment benefit of selinexor in heavily pretreated patients and support the notion that selinexor may overcome resistance to prior therapies, with no new safety concerns arising.
Keywords: UK; case series; objective response rate; progression‐free survival; safety; selinexor.
© 2024 The Author(s). eJHaem published by British Society for Haematology and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Rajkumar SV. Multiple myeloma: 2020 update on diagnosis, risk‐stratification and management. Am J Hematol. 2020;95:548–567. - PubMed
-
- Dimopoulos MA, Moreau P, Terpos E, Mateos MV, Zweegman S, Cook G, et al. Multiple myeloma: EHA‐ESMO Clinical Practice Guidelines for diagnosis, treatment and follow‐up. Ann Oncol. 2021;32:309–322. - PubMed
-
- Chari A, Vogl DT, Gavriatopoulou M, Nooka AK, Yee AJ, Huff CA, et al. Oral selinexor–dexamethasone for triple‐class refractory multiple myeloma. N Engl J Med. 2019;381:727‒738. - PubMed
-
- Syed YY. Selinexor: first global approval. Drugs. 2019;79:1485–1494. - PubMed
-
- Grosicki S, Simonova M, Spicka I, Pour L, Kriachok I, Gavriatopoulou M, et al. Once‐per‐week selinexor, bortezomib, and dexamethasone versus twice‐per‐week bortezomib and dexamethasone in patients with multiple myeloma (BOSTON): a randomised, open‐label, phase 3 trial. Lancet North Am Ed. 2020;396:1563–1573. - PubMed
LinkOut - more resources
Full Text Sources