This is a preprint.
Danicamtiv reduces myosin's working stroke but enhances contraction by activating the thin filament
- PMID: 39416013
- PMCID: PMC11482770
- DOI: 10.1101/2024.10.09.617269
Danicamtiv reduces myosin's working stroke but enhances contraction by activating the thin filament
Abstract
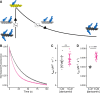
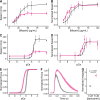
Heart failure is a leading cause of death worldwide, and even with current treatments, the 5-year transplant-free survival rate is only ~50-70%. As such, there is a need to develop new treatments for patients that improve survival and quality of life. Recently, there have been efforts to develop small molecules for heart failure that directly target components of the sarcomere, including cardiac myosin. One such molecule, danicamtiv, recently entered phase II clinical trials; however, its mechanism of action and direct effects on myosin's mechanics and kinetics are not well understood. Using optical trapping techniques, stopped flow transient kinetics, and in vitro reconstitution assays, we found that danicamtiv reduces the size of cardiac myosin's working stroke, and in contrast to studies in muscle fibers, we found that it does not affect actomyosin detachment kinetics at the level of individual crossbridges. We demonstrate that danicamtiv accelerates actomyosin association kinetics, leading to increased recruitment of myosin crossbridges and subsequent thin filament activation at physiologically-relevant calcium concentrations. Finally, we computationally model how the observed changes in mechanics and kinetics at the level of single crossbridges contribute to increased cardiac contraction and improved diastolic function compared to the related myotrope, omecamtiv mecarbil. Taken together, our results have important implications for the design of new sarcomeric-targeting compounds for heart failure.
Keywords: cardiac myosin; contractility; single molecule.
Conflict of interest statement
All experiments were conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest. M.J.G. discloses research funding from Edgewise Therapeutics on an unrelated project.
Figures




References
-
- Heidenreich P. A. et al. , 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol 79, 1757–1780 (2022). - PubMed
-
- Rogers J. G. et al. , Chronic mechanical circulatory support for inotrope-dependent heart failure patients who are not transplant candidates: results of the INTrEPID Trial. J Am Coll Cardiol 50, 741–747 (2007). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
