This is a preprint.
Catabolism of extracellular glutathione supplies amino acids to support tumor growth
- PMID: 39416022
- PMCID: PMC11482906
- DOI: 10.1101/2024.10.10.617667
Catabolism of extracellular glutathione supplies amino acids to support tumor growth
Abstract
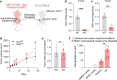
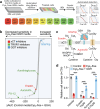
Restricting amino acids from tumors is an emerging therapeutic strategy with significant promise. While typically considered an intracellular antioxidant with tumor-promoting capabilities, glutathione (GSH) is a tripeptide of cysteine, glutamate, and glycine that can be catabolized, yielding amino acids. The extent to which GSH-derived amino acids are essential to cancers is unclear. Here, we find that GSH catabolism promotes tumor growth. We show that depletion of intracellular GSH does not perturb tumor growth, and extracellular GSH is highly abundant in the tumor microenvironment, highlighting the potential importance of GSH outside of tumors. We find supplementation with GSH can rescue cancer cell survival and growth in cystine-deficient conditions, and this rescue is dependent on the catabolic activity of γ-glutamyltransferases (GGTs). Finally, pharmacologic targeting of GGTs' activity prevents the breakdown of circulating GSH, lowers tumor cysteine levels, and slows tumor growth. Our findings indicate a non-canonical role for GSH in supporting tumors by acting as a reservoir of amino acids. Depriving tumors of extracellular GSH or inhibiting its breakdown is potentially a therapeutically tractable approach for patients with cancer. Further, these findings change our view of GSH and how amino acids, including cysteine, are supplied to cells.
Conflict of interest statement
Competing Interests Statement All authors declare no competing interests.
Figures



References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
