This is a preprint.
The invasion pore induced by Toxoplasma gondii
- PMID: 39416144
- PMCID: PMC11482919
- DOI: 10.1101/2024.10.11.617945
The invasion pore induced by Toxoplasma gondii
Update in
-
The invasion pore induced by Toxoplasma gondii.EMBO Rep. 2025 Oct;26(20):5009-5026. doi: 10.1038/s44319-025-00565-8. Epub 2025 Sep 19. EMBO Rep. 2025. PMID: 40973829 Free PMC article.
Abstract
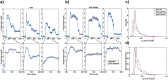
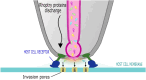
Obligate intracellular parasites invade host cells to survive. Following host cell contact, the apicomplexan Toxoplasma gondii injects proteins required for invasion into the host cell. Here, electrophysiological recordings of host cells acquired at sub-200 ms resolution allowed detection and analysis of a transient increase in host membrane conductance following exposure to Toxoplasma gondii. Transients always preceded invasion but parasites depleted of the moving junction protein RON2 generated transients without invading, ruling out a direct structural role for RON2 in generating the conductance pathway or restricting the diffusion of its components. Time-series analysis developed for transients and applied to the entire transient dataset (910,000 data points) revealed multiple quantal conductance changes in the parasite-induced transient, consistent with a rapid insertion, then slower removal, blocking, or inactivation of pore-like conductance steps. Quantal steps for RH had a principal mode with Gaussian mean of 0.26 nS, similar in step size to the apicomplexan protein translocon EXP2. Without RON2 the quantal mean was significantly different (0.19 nS). Because no invasion occurs without poration, the term 'invasion pore' is proposed.
Figures
References
-
- Aquilini E., Cova M. M., Mageswaran S. K., Dos Santos Pacheco N., Sparvoli D., Penarete-Vargas D. M., Najm R., Graindorge A., Suarez C., Maynadier M., Berry-Sterkers L., Urbach S., Fahy P. R., Guérin A. N., Striepen B., Dubremetz J. F., Chang Y. W., Turkewitz A. P., & Lebrun M. (2021). An Alveolata secretory machinery adapted to parasite host cell invasion. Nature microbiology, 6(4), 425–434. 10.1038/s41564-020-00854-z - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
