This is a preprint.
Gender- and Age-Based Characterization and Comparison of the Murine Primary Peritoneal Mesothelial Cell Proteome
- PMID: 39416176
- PMCID: PMC11482775
- DOI: 10.1101/2024.10.09.617441
Gender- and Age-Based Characterization and Comparison of the Murine Primary Peritoneal Mesothelial Cell Proteome
Abstract
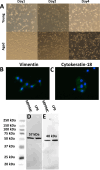
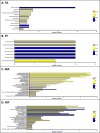
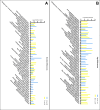
Organs in the abdominal cavity are covered by a peritoneal membrane, which is comprised of a monolayer of mesothelial cells (MC). Diseases involving the peritoneal membrane include peritonitis, primary cancer (mesothelioma), and metastatic cancers (ovarian, pancreatic, colorectal). These diseases have gender- and/or age-related pathologies; however, the impact of gender and age on the peritoneal MC is not well evaluated. To address this, we identified and characterized gender- and age-related differences in the proteomes of murine primary peritoneal MC. Primary peritoneal MC were isolated from young female (FY) or male (MY) mice (3-6 months) and aged female (FA) or male (MA) mice (20-23 months), lysed, trypsin digested using S-Traps, then subjected to bottom-up proteomics using an LC-Orbitrap mass spectrometer. In each cohort, we identified >1000 protein groups. Proteins were categorized using Gene Ontology and pairwise comparisons between gender and age cohorts were conducted. This study establishes baseline information for studies on peritoneal MC in health and disease at two physiologic age/gender points. Segregation of the data by gender and age could reveal novel factors to specific disease states involving the peritoneum. [This in vitro primary cell model has utility for future studies on the interaction between the mesothelium and foreign materials.].
Keywords: aging; mesothelial cell; mesothelium; peritoneal proteomics.
Conflict of interest statement
CONFLICT OF INTEREST The authors have no financial or commercial conflicts of interest to declare.
Figures



References
-
- Yung S., & Chan T.-M. (2001). Peritoneal mesothelial cells and the extracellular matrix. Nephrology (Carlton, Vic.), 6(6), 250–258. doi: 10.1046/j.1440-1797.2001.00081.x - DOI
-
- Nagy J. A., & Jackman R. W. (1998). Anatomy and Physiology of the Peritoneal Membrane. Seminars in dialysis, 11(1), 49–56. doi: 10.1111/j.1525-139X.1998.tb00210.x - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
