Perylene-derivative singlet exciton fission in water solution
- PMID: 39416301
- PMCID: PMC11472385
- DOI: 10.1039/d4sc04732j
Perylene-derivative singlet exciton fission in water solution
Erratum in
-
Correction: Perylene-derivative singlet exciton fission in water solution.Chem Sci. 2024 Dec 4;16(1):448. doi: 10.1039/d4sc90232g. eCollection 2024 Dec 18. Chem Sci. 2024. PMID: 39640029 Free PMC article.
Abstract
We provide direct evidence of singlet fission occurring with water-soluble compounds. We show that perylene-3,4,9,10-tetracarboxylate forms dynamic dimers in aqueous solution, with lifetimes long enough to allow intermolecular processes such as singlet fission. As these are transient dimers rather than stable aggregates, they retain a significant degree of disorder. We performed a comprehensive analysis of such dynamic assemblies using time-resolved absorption and fluorescence spectroscopy, nuclear magnetic resonance spectroscopy, and theoretical modelling, allowing us to observe the characteristic signatures of singlet fission and develop a model to characterize the different species observed. Our findings reveal that structure fluctuations within perylene-3,4,9,10-tetracarboxylate associations are key in favoring either singlet fission or charge separation. The efficiency of triplet formation is higher than 100%, and the disordered system leads to triplets living in the nanosecond time range.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures


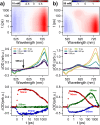





References
-
- Singh S. Jones W. J. Siebrand W. Stoicheff B. P. Schneider W. G. Laser generation of excitons and fluorescence in anthracene crystals. J. Chem. Phys. 1965;42:330–342. doi: 10.1063/1.1695695. - DOI
-
- Swenberg C. E. Stacy W. T. Bimolecular radiationless transitions in crystalline tetracene. Chem. Phys. Lett. 1968;2:327–328. doi: 10.1016/0009-2614(68)80087-9. - DOI
-
- Geacintov N. Pope M. Vogel F. Effect of magnetic field on the fluorescence of tetracene crystals: exciton fission. Phys. Rev. Lett. 1969;22:593–596. doi: 10.1103/PhysRevLett.22.593. - DOI
-
- Merrifield R. E. Avakian P. Groff R. P. Fission of singlet excitons into pairs of triplet excitons in tetracene crystals. Chem. Phys. Lett. 1969;3:155–157. doi: 10.1016/0009-2614(69)80122-3. - DOI
LinkOut - more resources
Full Text Sources
Research Materials

