Organ-on-chip technology: Opportunities and challenges
- PMID: 39416695
- PMCID: PMC11446384
- DOI: 10.1016/j.biotno.2024.01.001
Organ-on-chip technology: Opportunities and challenges
Abstract
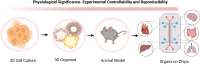
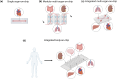
Organ-on-chip (OOC) technology is an innovative approach that reproduces human organ structures and functions on microfluidic platforms, offering detailed insights into intricate physiological processes. This technology provides unique advantages over conventional in vitro and in vivo models and thus has the potential to become the new standard for biomedical research and drug screening. In this mini-review, we compare OOCs with conventional models, highlighting their differences, and present several applications of OOCs in biomedical research. Additionally, we highlight advancements in OOC technology, particularly in developing multiorgan systems, and discuss the challenges and future directions of this field.
Keywords: 2D/3D culture models; Gut-on-Chip; Kidney-on-chip; Liver-on-chip; Lung-on-chip; Microfluidics; Organs-on-chips (OOCs).
© 2024 The Authors.
Conflict of interest statement
The authors declare no conflict of interest. Matthew Wook Chang is the Editor-in-Chief for Biotechnology Notes and was not involved in the editorial review or the decision to publish this article.
Figures

References
-
- Lin X., Wang T., Lu Y. Cell-free synthetic biology: orchestrating the machinery for biomolecular engineering. Biotechnology Notes. 2022;3:97–101.
-
- Krink N., Löchner A.C., Cooper H., Beisel C.L., Di Ventura B. Synthetic biology landscape and community in Germany. Biotechnology Notes. 2022;3:8–14.
Publication types
LinkOut - more resources
Full Text Sources

