Extracapsular extension risk assessment using an artificial intelligence prostate cancer mapping algorithm
- PMID: 39416757
- PMCID: PMC11479810
- DOI: 10.1002/bco2.421
Extracapsular extension risk assessment using an artificial intelligence prostate cancer mapping algorithm
Erratum in
-
Erratum.BJUI Compass. 2024 Dec 30;5(12):1324-1329. doi: 10.1002/bco2.482. eCollection 2024 Dec. BJUI Compass. 2024. PMID: 39744071 Free PMC article.
Abstract
Objective: The objective of this study is to compare detection rates of extracapsular extension (ECE) of prostate cancer (PCa) using artificial intelligence (AI)-generated cancer maps versus MRI and conventional nomograms.
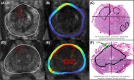
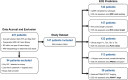
Materials and methods: We retrospectively analysed data from 147 patients who received MRI-targeted biopsy and subsequent radical prostatectomy between September 2016 and May 2022. AI-based software cleared by the United States Food and Drug Administration (Unfold AI, Avenda Health) was used to map 3D cancer probability and estimate ECE risk. Conventional ECE predictors including MRI Likert scores, capsular contact length of MRI-visible lesions, PSMA T stage, Partin tables, and the "PRedicting ExtraCapsular Extension" nomogram were used for comparison.Postsurgical specimens were processed using whole-mount histopathology sectioning, and a genitourinary pathologist assessed each quadrant for ECE presence. ECE predictors were then evaluated on the patient (Unfold AI versus all comparators) and quadrant level (Unfold AI versus MRI Likert score). Receiver operator characteristic curves were generated and compared using DeLong's test.
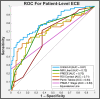
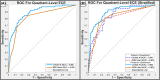
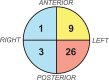
Results: Unfold AI had a significantly higher area under the curve (AUC = 0.81) than other predictors for patient-level ECE prediction. Unfold AI achieved 68% sensitivity, 78% specificity, 71% positive predictive value, and 75% negative predictive value. At the quadrant level, Unfold AI exceeded the AUC of MRI Likert scores for posterior (0.89 versus 0.82, p = 0.003), anterior (0.84 versus 0.80, p = 0.34), and all quadrants (0.89 versus 0.82, p = 0.002). The false negative rate of Unfold AI was lower than MRI in both the anterior (-60%) and posterior prostate (-40%).
Conclusions: Unfold AI accurately predicted ECE risk, outperforming conventional methodologies. It notably improved ECE prediction over MRI in posterior quadrants, with the potential to inform nerve-spare technique and prevent positive margins. By enhancing PCa staging and risk stratification, AI-based cancer mapping may lead to better oncological and functional outcomes for patients.
Keywords: MRI; artificial intelligence; extracapsular extension; fusion biopsy; prostate cancer.
© 2024 The Author(s). BJUI Compass published by John Wiley & Sons Ltd on behalf of BJU International Company.
Conflict of interest statement
Unfold AI is commercially available cancer mapping software provided by Avenda Health. Dr. Brisbane receives no financial compensation from Avenda Health. He had complete control of the data and supervised manuscript preparation. Dr. Sayre performed all statistical analyses. Dr. Priester and Dr. Mota were responsible for deriving AI‐based ECE risk prediction. Dr. Priester, Dr. Mota, and Mr. Shubert are employees at Avenda Health. Dr. Natarajan and Dr. Marks are co‐founders of Avenda Health.
Figures

References
-
- Preisser F, Cooperberg MR, Crook J, Feng F, Graefen M, Karakiewicz PI, et al. Intermediate‐risk prostate cancer: stratification and management. Eur Urol Oncol [Internet]. 2020;3(3):270–280. Available from: https://www.sciencedirect.com/science/article/pii/S2588931120300444, 10.1016/j.euo.2020.03.002 - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
