Efficacy, Safety, and Tolerability of Switching From Bictegravir/Emtricitabine/Tenofovir Alafenamide to Dolutegravir/Lamivudine Among Adults With Virologically Suppressed HIV: The DYAD Study
- PMID: 39416993
- PMCID: PMC11482008
- DOI: 10.1093/ofid/ofae560
Efficacy, Safety, and Tolerability of Switching From Bictegravir/Emtricitabine/Tenofovir Alafenamide to Dolutegravir/Lamivudine Among Adults With Virologically Suppressed HIV: The DYAD Study
Abstract
Background: In TANGO and SALSA, switching to dolutegravir/lamivudine (DTG/3TC) was noninferior to continuing a baseline regimen among adults who were treatment experienced, although few switched from bictegravir (B) / emtricitabine (F) / tenofovir alafenamide (TAF). Here, we present the efficacy and safety of switching to DTG/3TC as compared with continuing with B/F/TAF among adults with virologic suppression.
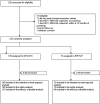
Methods: DYAD is an open-label clinical trial that randomized adults with HIV-1 RNA <50 copies/mL and no prior virologic failure (2:1) to switch to once-daily fixed-dose DTG/3TC or maintain B/F/TAF. The primary end point is the proportion with HIV-1 RNA ≥50 copies/mL at week 48 (Food and Drug Administration Snapshot algorithm, intention-to-treat exposed population, 6% noninferiority margin).
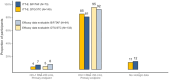
Results: Overall, 222 adults were randomized (16% women, 51% aged ≥50 years, 28% Black). At week 48, 6 (4%) with DTG/3TC and 5 (7%) with B/F/TAF had HIV-1 RNA ≥50 copies/mL (treatment difference, -2.8%; 95% CI, -11.4% to 3.1%), meeting noninferiority criteria. Through week 48, 18 participants (12 with DTG/3TC, 6 with B/F/TAF) met confirmed virologic withdrawal (CVW) criteria, and 2 of 18 had resistance: 1 with B/F/TAF developed M184M/I and G140G/S at week 12, and 1 with DTG/3TC had M184V at week 12. One participant with DTG/3TC and non-CVW developed M184V and K65R at week 12. Drug-related adverse events (AEs) and withdrawals due to AEs occurred in 31 (21%) and 6 (4%) participants with DTG/3TC and 2 (3%) and 0 participants with B/F/TAF, respectively.
Conclusions: Switching to DTG/3TC was noninferior to continuing B/F/TAF among adults with virologic suppression at week 48. Drug-related AEs and withdrawals were higher in the DTG/3TC arm, which is likely consistent with the open-label nature of this switch study.
Keywords: bictegravir/emtricitabine/tenofovir alafenamide; dolutegravir/lamivudine; randomized clinical trial; switch study; virologically suppressed.
© The Author(s) 2024. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest. C.-P. Rolle received research grants and honoraria from Gilead Sciences, ViiV Healthcare, and Janssen Infectious Diseases during the conduct of this study. F. H. received honoraria from Gilead Sciences, Merck, and AbbVie during the conduct of this study. E. D. received honoraria from Gilead Sciences during the conduct of this study. All other authors report no potential conflicts.
Figures

References
-
- Panel on Antiretroviral Guidelines for Adults and Adolescents . Guidelines for the use of antiretroviral agents in adults and adolescents with HIV. Department of Health and Human Services. Available at: https://clinicalinfo.hiv.gov/en/guidelines/adult-and-adolescent-arv. Accessed 16 July 2024.
-
- Gandhi RT, Bedimo R, Hoy JF, et al. Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2022 recommendations of the International Antiviral Society–USA Panel. JAMA 2023; 329:63–84. - PubMed
-
- Osiyemi O, De Wit S, Ajana F, et al. Efficacy and safety of switching to dolutegravir/lamivudine versus continuing a tenofovir alafenamide-based 3- or 4-drug regimen for maintenance of virologic suppression in adults living with human immunodeficiency virus type 1: results through week 144 from the phase 3, noninferiority TANGO randomized trial. Clin Infect Dis 2022; 75:975–86. - PMC - PubMed
-
- Bowe ZG, Shanmugam S, Hall-Thomsen H, Gubernick SI. The landscape and market for HIV therapies. Nat Rev Drug Discov 2024; 23:334–5. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

