This is a preprint.
Characterization of cell states in biliary tract cancers identifies mechanisms of therapeutic resistance in a phase II trial of DKN-01/nivolumab
- PMID: 39417106
- PMCID: PMC11483019
- DOI: 10.1101/2024.10.08.24315092
Characterization of cell states in biliary tract cancers identifies mechanisms of therapeutic resistance in a phase II trial of DKN-01/nivolumab
Abstract
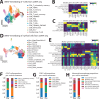
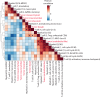
Biliary tract cancers demonstrate profound therapeutic resistance, and broadly effective therapies for refractory disease are lacking. We conducted a single-arm, second-line phase II trial combining DKN-01, a humanized monoclonal antibody targeting Dickkopf-1 (DKK-1), and nivolumab to treat patients with advanced biliary tract cancer (NCT04057365). No objective responses were seen. To identify mechanisms of treatment failure, we analyzed paired pre-treatment and on-treatment biopsies using scRNA-seq and constructed a detailed molecular classification of malignant and immune cells. We annotated five biliary tract cancer malignant cell states: classical, basal, mesenchymal, neural-like, and endothelial-like. Neural-like and endothelial-like states, which drive therapeutic resistance in other cancers, have not previously been described in BTC. Malignant cell states co-varied with distinct immune cell states, revealing diverse mechanisms of myeloid and T-cell mediated immune suppression, including M2 myeloid and terminally exhausted T cell programs that were induced by DKN-01/nivolumab. Here, we provide the first systematic classification of functionally annotated cell states in biliary tract cancer and provide new insight into resistance mechanisms to an immunotherapy combination that can inform the next generation of trials.
Conflict of interest statement
MP has served as a consultant for Third Rock Ventures. LP has served as a consultant for Astellas. DGD has received research funding support from Bayer, Bristol-Myers Squibb, Exelixis and Surface Oncology. NH holds equity in and advises Danger Bio/Related Sciences, is on the scientific advisory board of Repertoire Immune Medicines and CytoReason, owns equity in and has licensed patents to BioNTech and receives research funding from Bristol Myers Squibb and Calico Life Sciences. DTT has received consulting fees from ROME Therapeutics, Sonata Therapeutics, Leica Biosystems Imaging, PanTher Therapeutics, and abrdn. DTT is a founder and has equity in ROME Therapeutics, PanTher Therapeutics and TellBio, Inc., which is not related to this work. DTT is on the advisory board with equity for ImproveBio, Inc. DTT has received honorariums from Astellas, AstraZeneca, Moderna, and Ikena Oncology that are not related to this work. DTT receives research support from ACD-Biotechne, AVA LifeScience GmbH, Incyte Pharmaceuticals, Sanofi, and Astellas which was not used in this work. DTT’s interests were reviewed and are managed by Massachusetts General Hospital and Mass General Brigham in accordance with their conflict of interest policies. AM has served a consultant/advisory role for Third Rock Ventures, Asher Biotherapeutics, Abata Therapeutics, ManaT Bio, Flare Therapeutics, venBio Partners, BioNTech, Rheos Medicines and Checkmate Pharmaceuticals, is currently a part-time Entrepreneur in Residence at Third Rock Ventures, is an equity holder in ManaT Bio, Asher Biotherapeutics and Abata Therapeutics, and has received research funding support from Bristol-Myers Squibb. AM’s interests were reviewed and are managed by Massachusetts General Hospital and Mass General Brigham in accordance with their conflict of interest policies. All other authors have nothing to disclose.
Figures


References
-
- Mavros M.N., Economopoulos K.P., Alexiou V.G., and Pawlik T.M. (2014). Treatment and Prognosis for Patients With Intrahepatic Cholangiocarcinoma: Systematic Review and Meta-analysis. JAMA Surg. 149, 565–574. - PubMed
-
- Valle J., Wasan H., Palmer D.H., Cunningham D., Anthoney A., Maraveyas A., Madhusudan S., Iveson T., Hughes S., Pereira S.P., et al. (2010). Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N. Engl. J. Med. 362, 1273–1281. - PubMed
-
- Oh D.-Y., Ruth He A., Qin S., Chen L.-T., Okusaka T., Vogel A., Kim J.W., Suksombooncharoen T., Ah Lee M., Kitano M., et al. (2022). Durvalumab plus Gemcitabine and Cisplatin in Advanced Biliary Tract Cancer. NEJM Evid 1, EVIDoa2200015. - PubMed
-
- Kelley R.K., Ueno M., Yoo C., Finn R.S., Furuse J., Ren Z., Yau T., Klümpen H.-J., Chan S.L., Ozaka M., et al. (2023). Pembrolizumab in combination with gemcitabine and cisplatin compared with gemcitabine and cisplatin alone for patients with advanced biliary tract cancer (KEYNOTE-966): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 401, 1853–1865. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
