Tris(2-hydroxyethyl)ammonium-Based Protic "Ionic Liquids": Synthesis and Characterization
- PMID: 39417157
- PMCID: PMC11480899
- DOI: 10.1021/acs.jced.4c00024
Tris(2-hydroxyethyl)ammonium-Based Protic "Ionic Liquids": Synthesis and Characterization
Abstract
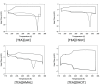
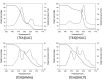
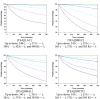
The proton transfer associated with the synthesis of protic ionic liquids (PILs) is often incomplete, meaning that the parent compounds may coexist with the ionic species. However, PILs are proposed for many applications as pure compounds without analysis of their ionicity. This work focuses on tris(2-hydroxyethyl)ammonium-based PILs with lactate, hydrogen succinate, hydrogen malate, and dihydrogen citrate anions. The interest of these anions lies in their low toxicity and capacity to disrupt the hydrogen-bonding network inherent to biopolymers. To improve current synthesis methods of this kind of PILs, which frequently lead to impurities derived from decomposition of reactants, working in the absence of solvents and at moderate temperatures is proposed. Through NMR studies, the ionicity of these systems was found to be low, from 20% to 86%, so the widely used term "ionic liquid" is not rigorous and must be used with caution. The un-ionized acid and base species coexist with the corresponding ionic forms, and this has to be considered in the studies involving these chemicals. The thermal characterization of the PILs was carried out. The influence of the anion on the thermal stability was found to be low. Isothermal thermogravimetric analysis showed that mass loss of these PILs starts at temperatures close to 350 K.
© 2024 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

References
-
- MacFarlane D. R.; Seddon K. R. Ionic Liquids-Progress on the Fundamental Issues. Aust. J. Chem. 2007, 60, 3–5. 10.1071/CH06478. - DOI
-
- Yuan X. L.; Zhang S. J.; Lu X. M. Hydroxyl Ammonium Ionic Liquids: Synthesis, Properties, and Solubility of SO2. J. Chem. Eng. Data 2007, 52, 596–599. 10.1021/je060479w. - DOI
-
- Petrović Z. D.; Čomić L.; Stefanović O.; Simijonović D.; Petrović V. P. Antimicrobial activity of the ionic liquids triethanolamine acetate and diethanolamine chloride, and their corresponding Pd(II) complexes. J. Mol. Liq. 2012, 170, 61–65. 10.1016/j.molliq.2012.03.009. - DOI
-
- Kondratenko Y.; Kochina T.; Fundamensky V.; Ignatyev I.; Panikorovskii T.; Nyanikova G. Triethanolammonium salicylate - Protic alkanolammonium ionic liquid. J. Mol. Liq. 2016, 221, 1218–1224. 10.1016/j.molliq.2016.06.085. - DOI
LinkOut - more resources
Full Text Sources
