Regulation of the NLRP3 inflammasome by autophagy and mitophagy
- PMID: 39417249
- PMCID: PMC12477832
- DOI: 10.1111/imr.13410
Regulation of the NLRP3 inflammasome by autophagy and mitophagy
Abstract
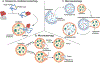
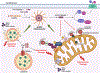
The NLRP3 inflammasome is a multiprotein complex that upon activation by the innate immune system drives a broad inflammatory response. The primary initial mediators of this response are pro-IL-1β and pro-IL-18, both of which are in an inactive form. Formation and activation of the NLRP3 inflammasome activates caspase-1, which cleaves pro-IL-1β and pro-IL-18 and triggers the formation of gasdermin D pores. Gasdermin D pores allow for the secretion of active IL-1β and IL-18 initiating the organism-wide inflammatory response. The NLRP3 inflammasome response can be beneficial to the host; however, if the NLRP3 inflammasome is inappropriately activated it can lead to significant pathology. While the primary components of the NLRP3 inflammasome are known, the precise details of assembly and activation are less well defined and conflicting. Here, we discuss several of the proposed pathways of activation of the NLRP3 inflammasome. We examine the role of subcellular localization and the reciprocal regulation of the NLRP3 inflammasome by autophagy. We focus on the roles of mitochondria and mitophagy in activating and regulating the NLRP3 inflammasome. Finally, we detail the impact of pathologic NLRP3 responses in the development and manifestations of pulmonary disease.
Keywords: NLRP3; caspase‐1; inflammasome; lung injury; mitochondria.
© 2024 John Wiley & Sons A/S. Published by John Wiley & Sons Ltd.
Conflict of interest statement
Conflict of Interest Statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

