The gut-brain axis in appetite, satiety, food intake, and eating behavior: Insights from animal models and human studies
- PMID: 39417406
- PMCID: PMC11483575
- DOI: 10.1002/prp2.70027
The gut-brain axis in appetite, satiety, food intake, and eating behavior: Insights from animal models and human studies
Abstract
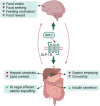
The gut-brain axis plays a pivotal role in the finely tuned orchestration of food intake, where both homeostatic and hedonic processes collaboratively control our dietary decisions. This interplay involves the transmission of mechanical and chemical signals from the gastrointestinal tract to the appetite centers in the brain, conveying information on meal arrival, quantity, and chemical composition. These signals are processed in the brain eventually leading to the sensation of satiety and the termination of a meal. However, the regulation of food intake and appetite extends beyond the realms of pure physiological need. Hedonic mechanisms, including sensory perception (i.e., through sight, smell, and taste), habitual behaviors, and psychological factors, exert profound influences on food intake. Drawing from studies in animal models and human research, this comprehensive review summarizes the physiological mechanisms that underlie the gut-brain axis and its interplay with the reward network in the regulation of appetite and satiety. The recent advancements in neuroimaging techniques, with a focus on human studies that enable investigation of the neural mechanisms underpinning appetite regulation are discussed. Furthermore, this review explores therapeutic/pharmacological strategies that hold the potential for controlling food intake.
Keywords: GLP‐1; brain imaging; fMRI; gut–brain axis; hedonic; homesostatic; obesity.
© 2024 The Author(s). Pharmacology Research & Perspectives published by British Pharmacological Society and American Society for Pharmacology and Experimental Therapeutics and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Salem V, Dhillo WS. Imaging in endocrinology: the use of functional MRI to study the endocrinology of appetite. Eur J Endocrinol. 2015;173(2):R59‐R68. - PubMed
-
- Florent V, Baroncini M, Jissendi‐Tchofo P, et al. Hypothalamic structural and functional imbalances in anorexia nervosa. Neuroendocrinology. 2020;110(6):552‐562. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

