Structure-based discovery of first inhibitors targeting the helicase activity of human PIF1
- PMID: 39417423
- PMCID: PMC11551755
- DOI: 10.1093/nar/gkae897
Structure-based discovery of first inhibitors targeting the helicase activity of human PIF1
Abstract
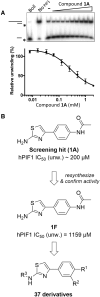
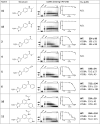
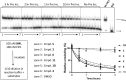
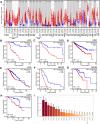
PIF1 is a conserved helicase and G4 DNA binding and unwinding enzyme, with roles in genome stability. Human PIF1 (hPIF1) is poorly understood, but its functions can become critical for tumour cell survival during oncogene-driven replication stress. Here we report the discovery, via an X-ray crystallographic fragment screen (XChem), of hPIF1 DNA binding and unwinding inhibitors. A structure was obtained with a 4-phenylthiazol-2-amine fragment bound in a pocket between helicase domains 2A and 2B, with additional contacts to Valine 258 from domain 1A. The compound makes specific interactions, notably through Leucine 548 and Alanine 551, that constrain conformational adjustments between domains 2A and 2B, previously linked to ATP hydrolysis and DNA unwinding. We next synthesized a range of related compounds and characterized their effects on hPIF1 DNA-binding and helicase activity in vitro, expanding the structure activity relationship (SAR) around the initial hit. A systematic analysis of clinical cancer databases is also presented here, supporting the notion that hPIF1 upregulation may represent a specific cancer cell vulnerability. The research demonstrates that hPIF1 is a tractable target through 4-phenylthiazol-2-amine derivatives as inhibitors of its helicase action, setting a foundation for creation of a novel class of anti-cancer therapeutics.
© The Author(s) 2024. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

