SWOG/NCI Phase II Dual Anti-CTLA-4/PD-1 Blockade in Rare Tumors: Nonepithelial Ovarian Cancer
- PMID: 39417692
- PMCID: PMC11737520
- DOI: 10.1158/1078-0432.CCR-24-0606
SWOG/NCI Phase II Dual Anti-CTLA-4/PD-1 Blockade in Rare Tumors: Nonepithelial Ovarian Cancer
Abstract
Purpose: The role of dual checkpoint inhibition (ipilimumab at 1 mg/kg intravenously every 6 weeks and nivolumab at 240 mg intravenously every 2 weeks) in advanced rare/ultrarare nonepithelial ovarian cancers is yet to be explored.
Patients and methods: Dual anti-CTLA-4 and anti-PD-1 blockade in rare tumor is a prospective, multicenter (1,016 US sites), multicohort, single-arm phase II trial conducted through the Early Therapeutics and Rare Cancer SWOG/NCI Committee, assessing ipilimumab (anti-CTLA-4; 1 mg/kg every 6 weeks) and nivolumab (anti-PD-1; 240 mg every 2 weeks) in adults with advanced nonepithelial ovarian cancers who lack beneficial standard therapy. The primary outcome was overall response rate [ORR; complete response (CR)/partial response (PR)]; secondary outcomes were progression-free survival (PFS), overall survival, clinical benefit rate [stable disease (SD) ≥6 months plus ORR], and toxicity.
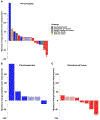
Results: Seventeen patients (median age: 64; number of prior therapies ranged from 0 to 8 with no immunotherapy exposure; eight granulosa, six carcinosarcomas, one Sertoli-Leydig, one yolk sac, and one Wolffian) were evaluated. In granulosa cell tumors, ORR was 25% (n = 2/8; one CR and one PR) and clinical benefit rate was 50% (n = 4/8); PFS was 58.3 (CR), 50.7+ (PR), 30.4 (SD), and 8.7 (SD) months. Median PFS was 3.5 months [95% confidence interval, 1.7-11.2 months]; median overall survival was 42.5 months (95% confidence interval, 10.1 months-not reached). One Sertoli-Leydig cell tumor showed a 22% regression (PFS, 11.2 months). Carcinosarcomas had no response. Three participants (18%) discontinued treatment due to grade 3 to grade 4 adverse events.
Conclusions: Ipilimumab-nivolumab shows activity in treatment-refractory granulosa cell tumors, with 25% (n = 2/8) of patients experiencing either CR or PR lasting more than 4 years.
©2024 American Association for Cancer Research.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

