Changes in Hepatic Steatosis Before and After Direct-Acting Antiviral Treatment in People With HIV and Hepatitis C Coinfection
- PMID: 39417816
- PMCID: PMC11793071
- DOI: 10.1093/infdis/jiae487
Changes in Hepatic Steatosis Before and After Direct-Acting Antiviral Treatment in People With HIV and Hepatitis C Coinfection
Abstract
Background: Both human immunodeficiency virus (HIV) and hepatitis C virus (HCV) infections increase the risk of hepatic steatosis (HS), which in turn contribute to the severity and progression of liver disease. Direct-acting antivirals (DAAs) can cure HCV but whether they reduce HS is unclear.
Methods: HS was assessed using the controlled attenuation parameter (CAP) and the Hepatic Steatosis Index (HSI) in participants coinfected with HIV and HCV from the Canadian Coinfection Cohort. Changes in HS, before, during, and after successful DAA treatment were estimated using generalized additive mixed models, adjusted for covariates measured prior to treatment (age, sex, duration of HCV infection, body mass index, diabetes, prior exposure to dideoxynucleosides, and hazardous drinking).
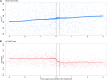
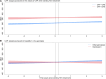
Results: In total, 431 participants with at least 1 measure of CAP or HSI before treatment were included. CAP steadily increased over time: adjusted annual slope 3.3 dB/m (95% credible interval [CrI], 1.6-4.9) before, and 3.9 dB/m (95% CrI, 1.9-5.9) after DAA treatment, irrespective of pretreatment CAP. In contrast, HSI changed little over time: annual slope 0.2 (95% CrI, -0.1 to 0.5) before and 0.2 (95% CrI, -0.1 to 0.5) after, but demonstrated a marked reduction during treatment -4.5 (95% CrI, -5.9 to -3.1).
Conclusions: When assessed by CAP, HS was unaffected by DAA treatment and steadily increased over time. In contrast, HSI did not appear to reflect changes in HS, with the decrease during treatment likely related to resolution of hepatic inflammation. Ongoing HS may pose a risk for liver disease in coinfected people cured of HCV.
Keywords: HIV-hepatitis C coinfection; Hepatic Steatosis Index; controlled attenuation parameter; direct-acting antivirals; hepatic steatosis.
© The Author(s) 2024. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest. M. B. K. reports grants for investigator-initiated studies from ViiV Healthcare, AbbVie, and Gilead; and consulting fees from ViiV Healthcare, Merck, AbbVie, and Gilead. G. S. has acted as speaker for Merck, Gilead, Abbvie, Novo Nordisk, and Pfizer; served as an advisory board member for Pfizer, Merck, Novo Nordisk, and Gilead; and has received unrestricted research funding from Theratecnologies, Inc and Merck. S. W. reports grants for investigator-initiated studies from ViiV Healthcare, Merck, and Gilead; and consulting fees and speaking at CME events for ViiV Healthcare, Merck, Gilead, and Jannsen. M. H. reports honoraria for speaking engagements or advisory boards and research funding from Gilead. C. C. reports speaker’s fees, advisor fees, and program support from Gilead Sciences, AbbVie, and ViiV Healthcare. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures


References
-
- Maurice JB, Patel A, Scott AJ, Patel K, Thursz M, Lemoine M. Prevalence and risk factors of nonalcoholic fatty liver disease in HIV-monoinfection. AIDS 2017; 31:1621–32. - PubMed
-
- Lonardo A, Adinolfi LE, Loria P, Carulli N, Ruggiero G, Day CP. Steatosis and hepatitis C virus: mechanisms and significance for hepatic and extrahepatic disease. Gastroenterology 2004; 126:586–97. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

