Novel use of Siltuximab in a patient with VEXAS Syndrome
- PMID: 39417832
- PMCID: PMC11971123
- DOI: 10.1007/s00277-024-06037-8
Novel use of Siltuximab in a patient with VEXAS Syndrome
Abstract
VEXAS syndrome (vacuoles, E1 enzyme, X-linked, autoinflammatory, somatic) is an increasingly recognized disorder that occurs due to somatic mutations of a ubiquitin-activating enzyme encoded by ubiquitin-like modifier activating enzyme 1 gene, UBA1. Clinical findings associated with VEXAS syndrome include recurrent fevers, polychondritis, periorbital edema, pleural effusions, myocarditis and/or pericarditis, hepatosplenomegaly, myelodysplastic syndrome, cytopenias, inflammatory arthritis, neutrophilic dermatosis, and deep venous thrombosis. Novel renal manifestations like interstitial nephritis are infrequent, and to our knowledge, acute renal failure due to C3 glomerulonephritis (C3GN) has not yet been reported. Overwhelming systemic inflammation can result in morbid end-organ damage and death. While there is no formal guideline or established protocol for its management, treatment of VEXAS syndrome with tocilizumab, an interleukin-6 (IL-6)-directed therapy, has been described in the literature. Here, we report a case of a 71-year-old male patient presenting with C3GN as an initial manifestation of VEXAS syndrome and explore the rationale for our approach to treatment with IL-6 blockade. Our patient was initially treated with two inpatient doses of tocilizumab with successful transition to siltuximab in the outpatient setting. He continues to benefit from ongoing siltuximab treatment for more than one year to date without any safety issues or relapse of VEXAS syndrome.
Keywords: UBA1; C3 glomerulonephritis; Case report; IL-6; Siltuximab; Systemic inflammation; VEXAS.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethical approval: The Office of Human Research Ethics at the University of North Carolina Chapel Hill has issued a determination that the research presented here does not constitute human subjects research as defined under federal regulations and does not require institutional review board (IRB) approval. Consent to publish: The participant has consented to the submission of the case report to the journal. Competing interests: Joshua Rivenbark: Research support from Agios; Manish K. Saha: Honoraria from Calliditas, Travere, ChemoCentryx, and Elsevier; Samuel M. Rubinstein: Ad-hoc consulting with EUSA pharma, Janssen, BMS, Sanofi, Lava Therapeutics; Beatriz Cáceres-Nazario and Stephanie Mathews have no competing interests.
Figures
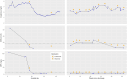
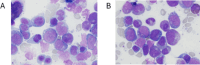
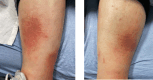
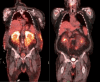

References
-
- Saad AJ, Patil MK, Cruz N, Lam CS, O’Brien C, Nambudiri VE (2024) VEXAS syndrome: a review of cutaneous findings and treatments in an emerging autoinflammatory disease. Exp Dermatol 33:e15050. 10.1111/exd.15050 - PubMed
-
- Bourbon E, Heiblig M, Gerfaud Valentin M et al (2021) Therapeutic options in VEXAS syndrome: insights from a retrospective series. Blood 137:3682–3684. 10.1182/blood.2020010177 - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

