Buprenorphine/Naloxone vs Methadone for the Treatment of Opioid Use Disorder
- PMID: 39418046
- PMCID: PMC11581542
- DOI: 10.1001/jama.2024.16954
Buprenorphine/Naloxone vs Methadone for the Treatment of Opioid Use Disorder
Abstract
Importance: Previous studies on the comparative effectiveness between buprenorphine and methadone provided limited evidence on differences in treatment effects across key subgroups and were drawn from populations who use primarily heroin or prescription opioids, although fentanyl use is increasing across North America.
Objective: To assess the risk of treatment discontinuation and mortality among individuals receiving buprenorphine/naloxone vs methadone for the treatment of opioid use disorder.
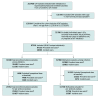
Design, setting, and participants: Population-based retrospective cohort study using linked health administrative databases in British Columbia, Canada. The study included treatment recipients between January 1, 2010, and March 17, 2020, who were 18 years or older and not incarcerated, pregnant, or receiving palliative cancer care at initiation.
Exposures: Receipt of buprenorphine/naloxone or methadone among incident (first-time) users and prevalent new users (including first and subsequent treatment attempts).
Main outcomes and measures: Hazard ratios (HRs) with 95% compatibility (confidence) intervals were estimated for treatment discontinuation (lasting ≥5 days for methadone and ≥6 days for buprenorphine/naloxone) and all-cause mortality within 24 months using discrete-time survival models for comparisons of medications as assigned at initiation regardless of treatment adherence ("initiator") and received according to dosing guidelines (approximating per-protocol analysis).
Results: A total of 30 891 incident users (39% receiving buprenorphine/naloxone; 66% male; median age, 33 [25th-75th, 26-43] years) were included in the initiator analysis and 25 614 in the per-protocol analysis. Incident users of buprenorphine/naloxone had a higher risk of treatment discontinuation compared with methadone in initiator analyses (88.8% vs 81.5% discontinued at 24 months; adjusted HR, 1.58 [95% CI, 1.53-1.63]), with limited change in estimates when evaluated at optimal dose in per-protocol analysis (42.1% vs 30.7%; adjusted HR, 1.67 [95% CI, 1.58-1.76]). Per-protocol analyses of mortality while receiving treatment exhibited ambiguous results among incident users (0.08% vs 0.13% mortality at 24 months; adjusted HR, 0.57 [95% CI, 0.24-1.35]) and among prevalent users (0.08% vs 0.09%; adjusted HR, 0.97 [95% CI, 0.54-1.73]). Results were consistent after the introduction of fentanyl and across patient subgroups and sensitivity analyses.
Conclusions and relevance: Receipt of methadone was associated with a lower risk of treatment discontinuation compared with buprenorphine/naloxone. The risk of mortality while receiving treatment was similar for buprenorphine/naloxone and methadone, although the CI estimate for the hazard ratio was wide.
Conflict of interest statement
Figures

References
-
- Substance Abuse and Mental Health Services Administration . TIP 63: Medications for Opioid Use Disorder. Substance Abuse and Mental Heath Services Administration; 2021.
-
- A Guideline for the Clinical Management of Opioid Use Disorder. British Columbia Centre on Substance Use; 2017.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

