Clinical Consequences of Delayed Gastric Emptying With GLP-1 Receptor Agonists and Tirzepatide
- PMID: 39418085
- PMCID: PMC11651700
- DOI: 10.1210/clinem/dgae719
Clinical Consequences of Delayed Gastric Emptying With GLP-1 Receptor Agonists and Tirzepatide
Erratum in
-
Correction to: "Clinical Consequences of Delayed Gastric Emptying With GLP-1 Receptor Agonists and Tirzepatide".J Clin Endocrinol Metab. 2025 Jul 10:dgaf387. doi: 10.1210/clinem/dgaf387. Online ahead of print. J Clin Endocrinol Metab. 2025. PMID: 40638456 No abstract available.
Abstract
Context: Glucagon-like peptide-1 (GLP-1) receptor agonists (RAs) are established therapeutics for type 2 diabetes and obesity. Among other mechanisms, they slow gastric emptying and motility of the small intestine. This helps to limit postprandial glycemic excursions and reduce chylomicron formation and triglyceride absorption. Conversely, motility effects may have detrimental consequences, eg, retained gastric contents at endoscopy or general anesthesia, potentially complicated by pulmonary aspiration or bowel obstruction.
Data acquisition: We searched the PubMed database for studies involving GLP-1RA therapy and adverse gastrointestinal/biliary events.
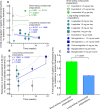
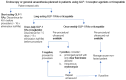
Data synthesis: Retained gastric contents at the time of upper gastrointestinal endoscopy are found more frequently with GLP-1 RAs but rarely are associated with pulmonary aspiration. Well-justified recommendations for the periprocedural management of GLP-1RAs (eg, whether to withhold these medications and for how long) are compromised by limited evidence. Important aspects to be considered are (1) their long half-lives, (2) the capacity of GLP-1 receptor agonism to slow gastric emptying even at physiological GLP-1 concentrations, (c) tachyphylaxis observed with prolonged treatment, and (d) the limited effect on gastric emptying in individuals with slow gastric emptying before initiating treatment. Little information is available on the influence of diabetes mellitus itself (ie, in the absence of GLP-1 RA treatment) on retained gastric contents and pulmonary aspiration.
Conclusion: Prolonged fasting periods regarding solid meal components, point-of-care ultrasound examination for retained gastric content, and the use of prokinetic medications like erythromycin may prove helpful and represent an important area needing further study to increase patient safety for those treated with GLP-1 RAs.
Keywords: GLP-1 receptor agonists; GLP-1/GIP dual receptor agonists; aspiration; gastric emptying; incretin mimetics; retained gastric content; tachyphylaxis.
© The Author(s) 2024. Published by Oxford University Press on behalf of the Endocrine Society.
Figures





Similar articles
-
Management of type 2 diabetes with the dual GIP/GLP-1 receptor agonist tirzepatide: a systematic review and meta-analysis.Diabetologia. 2022 Aug;65(8):1251-1261. doi: 10.1007/s00125-022-05715-4. Epub 2022 May 17. Diabetologia. 2022. PMID: 35579691 Free PMC article.
-
Gastrointestinal effects of GLP-1 receptor agonists: mechanisms, management, and future directions.Lancet Gastroenterol Hepatol. 2024 Oct;9(10):957-964. doi: 10.1016/S2468-1253(24)00188-2. Epub 2024 Aug 1. Lancet Gastroenterol Hepatol. 2024. PMID: 39096914 Review.
-
Implications of GLP-1 Agonists on Office-Based Sedation and General Anesthesia for Dentistry.Anesth Prog. 2025 Mar 12;72(1):52-58. doi: 10.2344/anpr-72-1-ANPR_Agonists. Anesth Prog. 2025. PMID: 40657828 Free PMC article. Review.
-
Endoscopy and anesthesia outcomes associated with glucagon-like peptide-1 receptor agonist use in patients undergoing outpatient upper endoscopy.Gastrointest Endosc. 2025 Aug;102(2):216-222. doi: 10.1016/j.gie.2025.01.004. Epub 2025 Jan 15. Gastrointest Endosc. 2025. PMID: 39824444
-
2025 ADS/ANZCA/GESA/NACOS clinical practice recommendations on the peri-procedural use of GLP-1/GIP receptor agonists.Anaesth Intensive Care. 2025 Aug 14:310057X251355288. doi: 10.1177/0310057X251355288. Online ahead of print. Anaesth Intensive Care. 2025. PMID: 40814081
Cited by
-
A narrative review of glucagon-like peptide-1 receptor agonists prior to deep sedation or general anesthesia.J Anesth Analg Crit Care. 2025 Mar 28;5(1):16. doi: 10.1186/s44158-025-00237-y. J Anesth Analg Crit Care. 2025. PMID: 40156058 Free PMC article. Review.
-
Obesity: Clinical Impact, Pathophysiology, Complications, and Modern Innovations in Therapeutic Strategies.Medicines (Basel). 2025 Jul 28;12(3):19. doi: 10.3390/medicines12030019. Medicines (Basel). 2025. PMID: 40843857 Free PMC article. Review.
-
Aspiration Risk During Endoscopic Procedures While on GLP-1 Agonist Therapy.Dig Dis Sci. 2025 Aug 9. doi: 10.1007/s10620-025-09307-1. Online ahead of print. Dig Dis Sci. 2025. PMID: 40783459
-
Gastroparesis, a diabetic complication causing further, even serious, complications: How to prevent its worsening?World J Gastroenterol. 2025 Jun 21;31(23):104932. doi: 10.3748/wjg.v31.i23.104932. World J Gastroenterol. 2025. PMID: 40575340 Free PMC article.
-
Unmasking Gastroparesis in Diabetes During Ramadan: Challenges and Management Strategies.J Clin Med. 2025 Mar 15;14(6):1997. doi: 10.3390/jcm14061997. J Clin Med. 2025. PMID: 40142805 Free PMC article. Review.
References
-
- International Diabetes Federation Guideline Development Group . Guideline for management of postmeal glucose in diabetes. Diabetes Res Clin Pract. 2014;103(2):256‐268. - PubMed
-
- Riddle MC. Basal glucose can be controlled, but the prandial problem persists- it’s the next target Diabetes Care. 2017;40(3):291‐300. - PubMed
-
- Monnier L, Lapinski H, Colette C. Contributions of fasting and postprandial plasma glucose increments to the overall diurnal hyperglycemia of type 2 diabetic patients: variations with increasing levels of HbA(1c). Diabetes Care. 2003;26(3):881‐885. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

