NK cell subsets define sustained remission in rheumatoid arthritis
- PMID: 39418106
- PMCID: PMC11623943
- DOI: 10.1172/jci.insight.182390
NK cell subsets define sustained remission in rheumatoid arthritis
Abstract
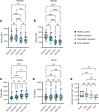
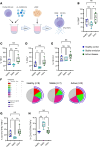
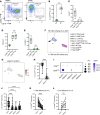
Rheumatoid arthritis (RA) is an immune-mediated, chronic inflammatory condition. With modern therapeutics and evidence-based management strategies, achieving sustained remission is increasingly common. To prevent complications associated with prolonged use of immunosuppressants, drug tapering or withdrawal is recommended. However, due to the lack of tools that define immunological remission, disease flares are frequent, highlighting the need for a more precision medicine-based approach. Utilizing high-dimensional phenotyping platforms, we set out to define peripheral blood immunological signatures of sustained remission in RA. We identified that CD8+CD57+KIR2DL1+ NK cells are associated with sustained remission. Functional studies uncovered an NK cell subset characterized by normal degranulation responses and reduced proinflammatory cytokine expression, which was elevated in sustained remission. Furthermore, flow cytometric analysis of NK cells from synovial fluid combined with interrogation of a publicly available single-cell RNA-Seq dataset of synovial tissue from active RA identified a deficiency of the phenotypic characteristics associated with this NK cell remission signature. In summary, we have uncovered an immune signature of RA remission associated with compositional changes in NK cell phenotype and function that has implications for understanding the effect of sustained remission on host immunity and distinct features that may define operational tolerance in RA.
Keywords: Autoimmunity; NK cells; Rheumatology; Tolerance.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

