The IL-4-IL-4Rα axis modulates olfactory neuroimmune signaling to induce loss of smell
- PMID: 39418114
- PMCID: PMC11804309
- DOI: 10.1111/all.16338
The IL-4-IL-4Rα axis modulates olfactory neuroimmune signaling to induce loss of smell
Abstract
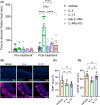
IL-4 and IL-13 have non-redundant effects in olfaction, with loss of smell in mice evoked only by intranasal administration of IL-4, but not IL-13. IL-4-evoked pathophysiological effects on olfaction is independent of compromised structural integrity of the olfactory neuroepithelium. IL-4-IL-4Rα signaling modulates neuronal crosstalk with immune cells, suggesting a functional link between olfactory impairment and neuroinflammation. Abbreviations: IL, interleukin; KO, knock-out; wk, week; WT, wild-type.
Background: Loss of smell is a part of the diagnostic criteria for CRSwNP. Although the mechanistic understanding is poor, inhibition of IL‐4Rα and IL‐4/IL‐13 signaling improves loss of smell in CRSwNP patients. In the present study, we compare the effects of IL‐4, IL‐13, and IL‐4Rα blockade on murine olfaction and identify the underlying pathophysiological mechanisms of loss of smell.
Methods: To evaluate the effects of IL‐4 and IL‐13 on olfactory function, we administered these cytokines intranasally to BALB/c mice for 5 consecutive days and assessed their latency to find hidden food. Calcium uptake assays were conducted to measure olfactory neuronal activity in vitro and ex vivo. We also performed histological analyses, proteomics, bulk RNA sequencing, and single‐cell RNA sequencing to assess the impact of IL‐4, IL‐13, and IL‐4Rα blockade on the olfactory epithelium and to identify potential molecular or cellular correlations with smell loss in human CRSwNP patients.
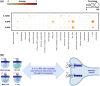
Results: Here, we provide evidence for non‐redundant effects of IL‐4 and IL‐13 in olfaction, with loss of smell in mice evoked by intranasal administration of IL‐4, not IL‐13. We demonstrate that an IL‐4–IL‐4Rα axis has a direct functional impact on murine olfactory sensory neurons and evokes inflammatory cell infiltration and pathophysiologic modulation of unique molecular signatures in olfactory epithelium without compromising structural integrity. Furthermore, single‐cell analysis of olfactory epithelium reveals that IL‐4–IL‐4Rα signaling modulates neuronal crosstalk with mast cells, macrophages, and NK cells, suggesting a functional link between olfactory impairment and neuroinflammation.
Conclusion: Collectively, this study suggests that an IL‐4–IL‐4Rα signaling axis plays a unique pathophysiological role in olfactory dysfunction via direct effect on neurons and modulation of neuroimmune interactions.
© 2024 Sanofi and Regeneron Pharmaceuticals. Allergy published by European Academy of Allergy and Clinical Immunology and John Wiley & Sons Ltd.
Conflict of interest statement
All authors are/were employees or officers of Sanofi or Regeneron Pharmaceuticals and may hold stock and/or stock options in their respective company.
Figures






References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

