Pediatric Diffuse Lung Disease in Infants: Imaging Findings and Histopathologic Correlation
- PMID: 39418186
- PMCID: PMC11580020
- DOI: 10.1148/rg.240022
Pediatric Diffuse Lung Disease in Infants: Imaging Findings and Histopathologic Correlation
Abstract
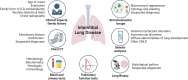
Childhood interstitial lung disease (chILD) encompasses a diverse group of genetic, infectious, and inflammatory conditions affecting infants and children. The recognition and understanding of these entities have highlighted the necessity for more accurate classification. This group of rare heterogeneous diseases comprises more than 200 different conditions and has a combined estimated prevalence of less than one patient per 100 000 children. Hence, a systematic diagnostic approach is crucial. This article describes a diagnostic approach for pediatric diffuse lung diseases in infancy, including an analysis of clinical presentations and imaging and histologic features to effectively distinguish among various chILD entities. Although they often have overlapping and nonspecific radiologic features, some chILD entities may exhibit typical imaging findings, resulting in a CT diagnosis or aiding in narrowing the differential diagnosis, thus guiding the clinician to the appropriate genetic tests, potentially limiting unnecessary biopsies. This approach aims to enhance the understanding and diagnosis of chILD in infants, thereby facilitating improved patient care.
© RSNA, 2024.
Conflict of interest statement
Figures
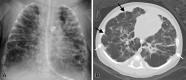
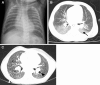
![Alveolar capillary dysplasia and pulmonary hypertension in an
8-month-old infant. (A, B) Coronal (A) and axial(B) CT images (lung windows)
show bilateral diffuse hazy ground-glass opacities (arrow), with
preferential involvement of the perihilar regions and lower lobes. There is
relative sparing of the upper lobes and the most peripheral regions of the
lungs (arrowheads) and dependent atelectasis in B. (C) Photomicrograph of
specimen shows slightly widened alveoli. The pulmonary artery (blue arrows)
shows medial hypertrophy, and there are abnormal thin-walled muscular veins
(green arrows) in the bronchovascular bundle. (Hematoxylin-eosin [H-E]
stain; original magnification, ×10.) (D) Higher-power photomicrograph
shows widened septa, with capillaries that are centrally located away from
the epithelial interface (arrows). (H-E stain; original magnification,
×20.) (E) Illustration shows abnormal positioning of pulmonary veins
and thickening of the interlobular septum.](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/6a1e/11580020/9426de59d0f1/rg.240022.fig5.gif)
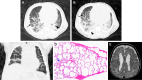
![Pulmonary interstitial glycogenosis in a 2-month-old full-term infant.
(A, B) Coronal (A) and axial (B) CT images (lung windows) show bilateral
patchy ground-glass airspace opacities (arrows) and centrilobular nodules in
the dependent upper and lower lobes. (C) Photomicrograph of specimen shows
mildly enlarged alveoli, but the septa are widened by bland mononuclear
cells with clear cytoplasms. There is minimal intra-alveolar material, and
type 2 pneumocytes are not prominent. (Hematoxylin-eosin stain; original
magnification, ×200 [objective, ×20].)](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/6a1e/11580020/9df65b2511b1/rg.240022.fig9.gif)
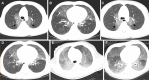
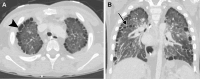
![ILD due to biallelic ABCA3 mutations in a 2-week-old neonate. (A, B)
Coronal (A) and axial (B) CT images (lung windows) show diffuse ground-glass
opacities with mild interlobular septal thickening (arrow). (C)
Photomicrograph of specimen shows widened alveolar septa with a combination
of smooth muscle, inflammatory cells, and fibroblasts. Most septa are lined
with reactive type 2 pneumocytes, and there are foci of intra-alveolar
macrophages. Intra-alveolar surfactant material is not present in this
field. Note that lung biopsy was performed before the availability of
genetic results because of clinical concern for ACDMPV. (Hematoxylin-eosin
stain; original magnification, ×100 [×10
objective].)](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/6a1e/11580020/cac02f4ed037/rg.240022.fig11.gif)
References
-
- Kurland G , Deterding RR , Hagood JS , et al. ; American Thoracic Society Committee on Childhood Interstitial Lung Disease (chILD) and the chILD Research Network . An official American Thoracic Society clinical practice guideline: classification, evaluation, and management of childhood interstitial lung disease in infancy . Am J Respir Crit Care Med 2013. ; 188 ( 3 ): 376 – 394 . - PMC - PubMed
-
- Schapiro AH , Baker ML , Rattan MS , Crotty EJ . Childhood interstitial lung disease more prevalent in infancy: a practical review . Pediatr Radiol 2022. ; 52 ( 12 ): 2267 – 2277 . - PubMed
-
- Casey AM , Deterding RR , Young LR , Fishman MP , Fiorino EK , Liptzin DR . Overview of the ChILD Research Network: A roadmap for progress and success in defining rare diseases . Pediatr Pulmonol 2020. ; 55 ( 7 ): 1819 – 1827 . - PubMed
-
- Liang T , Vargas SO , Lee EY . Childhood interstitial (diffuse) lung disease: pattern recognition approach to diagnosis in infants . AJR Am J Roentgenol 2019. ; 212 ( 5 ): 958 – 967 . - PubMed
-
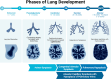
- Plosa E, Sucre J. Lung Development. Avery’s Diseases of the Newborn. 11th ed. Elsevier, 2024; 535–547.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

