The RECONCILE study protocol: Exploiting image-based risk stratification in early prostate cancer to discriminate progressors from non-progressors (RECONCILE)
- PMID: 39418289
- PMCID: PMC11486392
- DOI: 10.1371/journal.pone.0295994
The RECONCILE study protocol: Exploiting image-based risk stratification in early prostate cancer to discriminate progressors from non-progressors (RECONCILE)
Abstract
Introduction: RECONCILE (ClinicalTrials.gov:NCT04340245) will identify molecular and radiomic markers associated with clinical progression and radiological progression events in a cohort of localised, newly diagnosed Gleason 3 + 4 tumours. Molecular markers will be correlated against standard of care MRI-targeted histology and oncological outcomes.
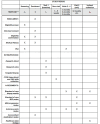
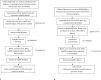
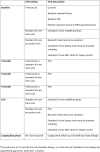
Methods: RECONCILE is an ethics approved (20/LO/0366) single centre, prospective, longitudinal, observational cohort study of recently diagnosed (within 12 months), organ-confined Gleason 3 + 4 cancers (MCCL ≤10mm) currently under active surveillance. 60 treatment-naïve participants with a concordant MRI lesion (Likert score 4 or 5) and PSA ≤ 15 ng/ml will be recruited. Blood, urine and targeted prostate tissue cores will be subject to next generation sequencing at baseline and one year in all participants. Semen will be collected from a specified sub-population. Baseline and interval MR images will be extracted from standard of care prostate MRI ahead of radiomic analysis. Data extracted from radiological and biological samples will be used to derive the association of molecular change and radiological progression, the primary outcome of the study. To compensate for spatial intratumoral heterogeneity and inherent sampling bias, a molecular index will be derived for each participant using the molecular profile of tumour tissue at both baseline (MolBL) and one year (MolFU). We will extract a ΔMolBL:MolFU score for each participant. Molecular progression will be defined as a MolBL:MolFU score >95% CI of the combined ΔMolBL scores. Radiological progression is defined as a PRECISE score of 4 or 5. The study is powered to detect an association with a statistical power of 80%.
Results: Recruitment began in July 2020 (n = 62). To date, 37 participants have donated tissue for analysis.
Conclusion: We have designed and implemented a prospective, longitudinal study to evaluate the underlying molecular landscape of intermediate risk, MR-visible prostate tumours. Recruitment is ongoing.
Copyright: © 2024 Marsden et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
I have read the journal’s policy and the authors of this manuscript have the following competing interests: Mark Emberton serves as a consultant/educator/trainer to Sonacare Inc., Exact Imaging, Angiodynamics Inc and Profound Medical. Acknowledgements - Mark Emberton receives research support from the United Kingdom’s National Institute of Health Research (NIHR) UCLH/UCL Biomedical Research Centre. Caroline M Moore receives funding from the Prostate Cancer UK, Movember, the Medical Research Council, Cancer Research UK and the NIHR. She receives fees for HIFU proctoring from SonaCare. She has received speaker fees from Astellas, and Jannsen. She carries out research into photodyanamic therapy supported by Spectracure. Shonit Punwani receives research support from the United Kingdom’s National Institute of Health Research (NIHR) UCLH/UCL Biomedical Research Centre. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures
References
-
- UK CR. Cancer screening and diagnosis statistics.
-
- Excellence NIfHaC. NICE guideline [NG131]: Prostate cancer: diagnosis and management May 201 2019. [Available from: https://www.nice.org.uk/guidance/ng131. - PubMed
-
- Mottet (Chair) PCV-c RCNvdB N., Briers (Patient Representative) E, De Santis M, Fanti S, Gillessen S, et al.. Willemse. EAU Prostate Cancer Guideline 2019. 2019.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous