Multiclass classification of Autism Spectrum Disorder, attention deficit hyperactivity disorder, and typically developed individuals using fMRI functional connectivity analysis
- PMID: 39418298
- PMCID: PMC11486369
- DOI: 10.1371/journal.pone.0305630
Multiclass classification of Autism Spectrum Disorder, attention deficit hyperactivity disorder, and typically developed individuals using fMRI functional connectivity analysis
Abstract
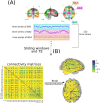
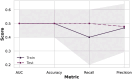
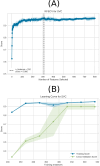
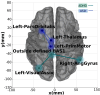
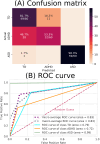
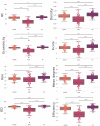
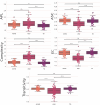
Neurodevelopmental conditions, such as Autism Spectrum Disorder (ASD) and Attention Deficit Hyperactivity Disorder (ADHD), present unique challenges due to overlapping symptoms, making an accurate diagnosis and targeted intervention difficult. Our study employs advanced machine learning techniques to analyze functional magnetic resonance imaging (fMRI) data from individuals with ASD, ADHD, and typically developed (TD) controls, totaling 120 subjects in the study. Leveraging multiclass classification (ML) algorithms, we achieve superior accuracy in distinguishing between ASD, ADHD, and TD groups, surpassing existing benchmarks with an area under the ROC curve near 98%. Our analysis reveals distinct neural signatures associated with ASD and ADHD: individuals with ADHD exhibit altered connectivity patterns of regions involved in attention and impulse control, whereas those with ASD show disruptions in brain regions critical for social and cognitive functions. The observed connectivity patterns, on which the ML classification rests, agree with established diagnostic approaches based on clinical symptoms. Furthermore, complex network analyses highlight differences in brain network integration and segregation among the three groups. Our findings pave the way for refined, ML-enhanced diagnostics in accordance with established practices, offering a promising avenue for developing trustworthy clinical decision-support systems.
Copyright: © 2024 Alves et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
EpilabKI is funded through the Bavarian stated Ministery for Sciences and the Arts research. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures




References
-
- Mahone EM, Warschausky S, Zabel TA. Introduction to the JINS special issue: Neurodevelopmental disorders. Journal of the International Neuropsychological Society. 2018;24(9):893–895. doi: 10.1017/S1355617718000905 - DOI
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

