Eighteen-Month Hybrid Closed-Loop Use in Very Young Children With Type 1 Diabetes: A Single-Arm Multicenter Trial
- PMID: 39418532
- PMCID: PMC11655413
- DOI: 10.2337/dc24-1313
Eighteen-Month Hybrid Closed-Loop Use in Very Young Children With Type 1 Diabetes: A Single-Arm Multicenter Trial
Abstract
Objective: We aimed to evaluate the longer-term safety and efficacy of hybrid closed-loop (CL) therapy in very young children with type 1 diabetes (T1D).
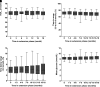
Research design and methods: Following a 16-week multinational, randomized crossover trial comparing hybrid CL with sensor-augmented pump (SAP) therapy in 74 very young children aged 1-7 years with T1D, participants were invited to an extension phase using CL for a further 18 months. Outcomes were compared with the primary-phase SAP period and primary-phase CL period.
Results: After the primary study phase, 60 participants were eligible to enroll in the extension. Of these, 49 consented (mean ± SD age 6.6 ± 1.5 years) to continue use of CL for 18 months. Percentage time in range (TIR) 3.9-10.0 mmol/L was 8.4 percentage points (95% CI 6.7-10.1; P < 0.001) higher, while HbA1c was 0.4% ([5.0 mmol/mol], 95% CI 0.3-0.6 [3.7-6.2]; P < 0.001) lower during the CL extension phase compared with primary-phase SAP period. At 18 months, mean HbA1c was 6.7 ± 0.5% and TIR was 70 ± 7%, compared with 6.7 ± 0.5% and 71 ± 6% in the primary-phase CL period. Time in hypoglycemia (<3.9 mmol/L) was similar between CL extension phase and both primary-phase SAP (P = 0.31) and CL periods (P = 0.70). There were two severe hypoglycemia events and one other serious adverse event during the extension phase. One unexpected serious adverse device effect occurred.
Conclusions: Use of the Cambridge hybrid CL system led to sustained improvements in glycemic control lasting more than 18 months in very young children with T1D.
© 2024 by the American Diabetes Association.
Conflict of interest statement
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

