Access barriers to anti-CD19+ CART therapy for NHL across a community transplant and cellular therapy network
- PMID: 39418599
- PMCID: PMC11846600
- DOI: 10.1182/bloodadvances.2024014171
Access barriers to anti-CD19+ CART therapy for NHL across a community transplant and cellular therapy network
Abstract
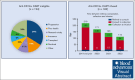
We analyzed access barriers to anti-CD19+ chimeric antigen receptor T cells (CARTs) for non-Hodgkin lymphoma (NHL) within a community-based transplant and cell therapy network registry. A total of 357 intended recipients for approved anti-CD19+ CARTs were identified between 2018 to 2022. The median age at referral was 61 years; referral years were 2018 (4%), 2019 (14%), 2020 (18%), 2021 (26%), and 2022 (38%). Diagnoses included diffuse large B cell (69%), follicular (13%), follicular/large (7%), mantle cell (4%), or other (7%). Axicabtagene ciloleucel (62%), tisagenlecleucel (16%), brexucabtagene autoleucel (13%), and lisocabtagene maraleucel (9 %) were infused into 182 patients. The median durations between referral to consultation, consultation to apheresis, and collection to infusion were 11, 107, and 32 days, respectively. The median duration from consultation to CART infusion declined steadily from 207 days in 2019 to 108 days in 2022 (P < .0001). A total of 124 patients (41%) did not receive CART, mostly for disease progression (34%) or poor health (15%). Multivariable logistic regression showed no significant differences in demographic, financial, or social determinants compared with those receiving CART. Notably, the proportion of ineligible patients declined from 53% in 2018-2020 to 34% by 2021-2022 (P = .001). In conclusion, 41% of community patients were unable to access timely CART therapy, mostly due to attrition from disease-related causes, and the overall time to infusion exceeded 4 months. Time to infusion and the proportion receiving CARTs improved over time. Reducing time to apheresis, early referral, and attention to salvage/bridging strategies are necessary.
© 2025 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: All authors report employment with HCA Healthcare.
Figures

References
-
- Pantin J, Battiwalla M. Upsetting the apple CAR-T (chimeric antigen receptor T-cell therapy) - sustainability mandates USA innovation. Br J Haematol. 2020;190(6):851–853. - PubMed
-
- Hoffmann MS, Hunter BD, Cobb PW, Varela JC, Munoz J. Overcoming barriers to referral for chimeric antigen receptor T cell therapy in patients with relapsed/refractory diffuse large B cell lymphoma. Transplant Cell Ther. 2023;29(7):440–448. - PubMed
-
- Locke FL, Miklos DB, Jacobson CA, et al. Axicabtagene ciloleucel as second-line therapy for large B-cell lymphoma. N Engl J Med. 2022;386(7):640–654. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

