Gene therapy in transfusion-dependent non-β0/β0 genotype β-thalassemia: first real-world experience of beti-cel
- PMID: 39418614
- PMCID: PMC11732601
- DOI: 10.1182/bloodadvances.2024014104
Gene therapy in transfusion-dependent non-β0/β0 genotype β-thalassemia: first real-world experience of beti-cel
Abstract
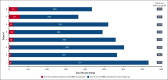
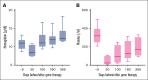
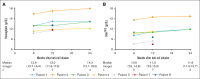
Gene addition and editing strategies for transfusion-dependent β-thalassemia have gained momentum as potentially curative treatment options, with studies showcasing their efficacy and safety. We report, to our knowledge, the first real-world application of betibeglogene autotemcel (beti-cel; Zynteglo) during its period of active license in Europe from January 2020 to March 2022 for patients aged ≥12 years without a β0/β0 genotype and without a HLA-matched sibling donor, before beti-cel marketing authorization was withdrawn by its holder because of nonsafety reasons. Among 15 screened patients, 4 opted out for fertility and safety concerns, 2 were excluded because of marked hepatic siderosis, and 1 had apheresis collection failure. Eight patients received beti-cel after busulfan myeloablative conditioning, all achieving transfusion independence within 8 to 59 days, with posttreatment hemoglobin levels ranging from 11.3 to 19.3 g/dL. No deaths occurred, but acute toxicity mirrored busulfan's known effects. Posttreatment platelet management faced challenges because of HLA-antibodies in 3 patients. Monitoring up to month 24 revealed pituitary-gonadal endocrine dysfunction in all 3 female and in 2 of 5 male patients. Additionally, we observed unexpected posttreatment sequelae: 1 patient developed polycythemia that could not be explained by known genetic or acquired mechanisms, 1 patient developed posttreatment depression and anxiety prohibiting her from returning to work, and 1 patient developed fatigue severely compromising both quality of life and work capacity. This real-world experience corroborates beti-cel's efficacy and safety and provides information on adverse events observed during real-world use of the therapy.
© 2024 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: A.K. received research support and royalties from bluebird bio, Inc. H.T. and G.T. are employees of, and hold stock/stock options in, bluebird bio. J.K. is a consultant for bluebird bio, Vertex, Novartis, and Pfizer, and receives research support from Pfizer, Novo Nordisk, Novartis, and Deutsche Kinderkrebsstiftung. The remaining authors declare no competing financial interests.
Figures
References
-
- Taher AT, Musallam KM, Cappellini MD. β-Thalassemias. N Engl J Med. 2021;384(8):727–743. - PubMed
-
- Thein SL. Pathophysiology of beta thalassemia--a guide to molecular therapies. Hematology Am Soc Hematol Educ Program. 2005;2005(1):31–37. - PubMed
-
- Scalone L, Mantovani LG, Krol M, et al. Costs, quality of life, treatment satisfaction and compliance in patients with β-thalassemia major undergoing iron chelation therapy: the ITHACA study. Curr Med Res Opin. 2008;24(7):1905–1917. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials