Global health 2050: the path to halving premature death by mid-century
- PMID: 39419055
- PMCID: PMC12320876
- DOI: 10.1016/S0140-6736(24)01439-9
Global health 2050: the path to halving premature death by mid-century
Erratum in
-
Department of Error.Lancet. 2024 Nov 9;404(10465):1814. doi: 10.1016/S0140-6736(24)02360-2. Epub 2024 Oct 25. Lancet. 2024. PMID: 39489904 No abstract available.
Abstract
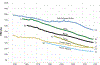
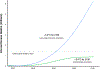
Global health 2050 (GH2050), a new report from the Lancet Commission on Investing in Health, finds that dramatic improvements in human welfare are achievable by mid-century with focused health investments. By 2050, countries that choose to do so can halve their probability of premature death (PPD)—the probability of dying before age 70—from their pre-pandemic level in 2019. We call this goal “50 by 50”: a 50% reduction in PPD by 2050. The interventions for achieving “50 by 50” will also reduce morbidity and disability at all ages.
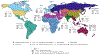
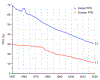
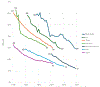
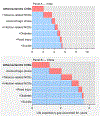
Historical experience and continued scientific advance indicate that this is a feasible aspiration. Eight of the 30 most populous countries reduced their PPD over the last decade at a rate that would halve PPD before 2050, including countries as diverse as Bangladesh, Iran, Tanzania, and Turkey. These focused gains can be achieved relatively early on the pathway to full universal health coverage (UHC).
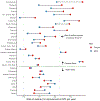
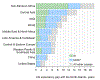
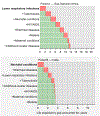
The path to achieving “50 by 50” runs through control of a remarkably narrow set of just 15 conditions. For currently high mortality countries, eight infectious diseases and maternal conditions are the highest priority. Seven clusters of noncommunicable diseases and injuries are important everywhere and addressing them will prove central to achieving “50 by 50” in most countries with lower initial levels of mortality.
Focused attention to health system strengthening (HSS) for primary care and first level hospitals will generate capacity to better tackle the 15 priority conditions and will be a critical step on the way to improving capacity to address all the conditions in a UHC package. Packaging interventions into 19 modules (e.g., a childhood immunization module, a module on cardiovascular disease prevention and low-cost, widely available treatment) will address the 15 priority conditions. Adopting this focused approach also invests in key areas of HSS and addresses major morbidities, such as psychiatric illness, not already covered by mortality-reducing interventions. Value for money can be assessed through a two-step process: technical cost effectiveness to assess how best to achieve module-specific goals (e.g., reduction in child mortality, reduction in cardiovascular mortality) and political evaluation of trade-offs in investing in expanding module coverage.
In many countries seeking reform, standard budgetary mechanisms have failed to successfully reorient systems toward priority interventions that improve health. This mechanism of blanket budget transfers from ministries of finance to ministries of health has not been fit to support such reorientation. The Commission concluded that this problem could be addressed by directing a substantial and increasing fraction of budget transfers to making available and affordable the specific drugs, vaccines, diagnostics, and other commodities that are currently available for control of the 15 priority conditions. Drug availability and affordability will typically require four complementary components: (i) redirecting general budget transfers to line item transfers (subsidies) for specific priority drugs; (ii) centralized procurement by government (or perhaps internationally); (iii) procurement in sufficient volumes to ensure availability when needed; and (iv) use and strengthening of existing supply chains, public and private.
Of the many intersectoral policies that governments can adopt to help achieve “50 by 50,” tobacco control is by far the most important, given the number of deaths caused by tobacco and the established and improving capacity of governments to implement tobacco policy. A high level of tobacco taxation is essential, and valuable in the short to medium term for public finance, and should be accompanied by a package of other effective tobacco control policies.
Background research conducted for the Commission points to exceptionally high ongoing levels of mortality risk from pandemics. Country performance against COVID-19 varied greatly, although eventual vaccine availability attenuated, but far from eliminated, this variability by the end of COVID-19’s emergency phase. National implementation of public health fundamentals—early action, isolation of infected individuals, quarantining of those exposed, and social and financial support for people isolating or quarantining—accounted for much of the success of the best-performing nations, such as Japan. In the next pandemic, these fundamentals will help to avert mortality while waiting for vaccine development and deployment.
The conclusions above are primarily aimed at national governments. Our final conclusion is aimed at the development assistance community. We conclude that such assistance should focus on two broad purposes. The first is to provide direct financial and technical support to countries with the least resources—to help develop health systems to better control diseases. The second is to finance global public goods, including strengthening data systems; reducing the development and spread of antimicrobial resistance; preventing and responding to pandemics; fostering global health leadership and advocacy; identifying and spreading best practices; and developing and deploying new health technologies. For both purposes, focusing efforts on the 15 priority conditions would best contribute to “50 by 50.” A decade ago, there were no malaria vaccines and the only available tuberculosis vaccine had low efficacy. Today, two partially successful malaria vaccines have been approved and three promising tuberculosis vaccine candidates are in late stage trials. These successes exemplify the enormous value in funding development of new medicines, vaccines, diagnostics, and operational research against the 15 priority conditions.
The prize of “50 by 50,” with an interim milestone of “30 by 2035” (a 30% reduction in PPD by 2035), remains a prize within reach. The most efficient route is to focus resources against a narrow set of conditions and scale up financing to develop and deploy new health technologies. Our economic analyses have shown that the value of achievable mortality declines remains high and indeed is often a substantial fraction of the value of gains in gross domestic product. Today, the case is better than ever for the value of investing in health for reducing mortality and morbidity, alleviating poverty, growing economies, and improving human welfare.
Conflict of interest statement
Declaration of interests OA declares consulting fees from the Asian Development Bank, WHO, the World Bank, and Pharos Global Health Advisors and speaker's fees from Pfizer. SA declares research grants from the US National Institutes of Health (NIH R01 R01DK127138, NIH R21MD019394, and NIH U01AI169477); consulting fees from Travere Therapeutics, Vera Therapeutics, and Mendara; support for travel or attending meetings from Travere Therapeutics; unpaid leadership or fiduciary roles with the International Society of Nephrology, the Kidney Health Initiative, and American Nephrologists of Indian Origin; and receipt of assay materials for work conducted under U01AI169477 from Abbott Laboratory and Ascend Laboratory. SFB declares consulting fees from the Serum Institute of India, Micron Biomedical, VAXCO, Global Health Investment Corporation, Brown University, Gavi, the Vaccine Alliance, and SICPA; payment or honoraria from University of California Press; support for attending meetings or travel from UN Office for Project Services and STOP TB, Gavi, SICPA, and Serum Life Sciences; participation on data safety monitoring or advisory boards for CEPI, COVAX, and Gavi; membership of the board of PHARE BIO and of the strategic oversight board of Apriori Bio; and stock or stock options in VAXCO and Apriori Bio. SMB declares that two graduate students reporting to him received support from the University of Bergen for work on pandemic preparedness as part of the 4th edition of the Disease Control Priorities Project; received support for travel from the University of Bergen, the Japan International Cooperation Agency, and the AIDS Healthcare Foundation; and is a board member for HopeLab and the Bay Area Global Health Alliance. SB declares research support from the University of Bergen and consulting fees from the World Bank. FB declares travel support from the Partnership for Maternal, Newborn and Child Health and Fondation Botnar and is chair of the Governance and Ethics Committee for the Partnership for Maternal, Newborn and Child Health, international advisory board chair of the UN University International Institute for Global Health, co-chair of the Lancet Commission on Gender-Based Violence and the Maltreatment of Young People, interim board chair of Fondation Botnar, a member of the Lancet Future of Neonatology Commission, and a member of the Lancet and Chatham House Commission on Universal Health. EG-P declares consulting fees from the International Monetary Fund and the World Bank, is board chair of Aceso Global, and has participated in advisory committees for Roche and Medtronic. WM declares research support to her institution from WHO, the Bill & Melinda Gates Foundation, the Pfizer Foundation, the Open Society Foundation, the Hilton Foundation, and the Rockefeller Foundation, and an unpaid role as a member of the Research Committee of the Consortium of Universities for Global Health. OO is a member of the Africa Centres for Disease Control and Prevention Health Economics and Financing Programme Advisory Board and a member of the Partnership for Maternal, Newborn and Child Health Economics and Financing Working Group. AP-M is a member of the board of the Global Alliance for TB Drug Development and Iliad Biotechnologies and a member of the Cabrini Global Health Commission, and has stock or stock options in Iliad Biotechnologies. DW declares a grant from the Research Council of Norway Centre of Excellence. GY declares research funding from WHO, the Gates Foundation, the Carnegie Corporation of New York, the UN Economic and Social Commission for Asia and the Pacific, and the Economic and Social Research Council, is co-chair of the Economics and Finance Working Group of the Partnership for Maternal, Newborn and Child Health, and has served as a paid adviser to the evaluation of Partners for a Malaria-Free Zambia Program of Scale (conducted by Metrics 4 Management). All other authors declare no competing interests.
Figures
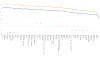

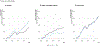

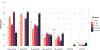



References
-
- World Bank W. World Development Report [Internet]. 1993. Available from: https://elibrary.worldbank.org/doi/abs/10.1596/0-1952-0890-0 - DOI
-
- Jamison DT, Summers LH, Alleyne G. Global health 2035: a world converging within a generation. Lancet. 2013;382:1898–955. - PubMed
-
- Watkins DA, Yamey G, Schäferhoff M. Alma Ata at 40 years: reflections from the Lancet Commission on Investing in Health. Lancet. 2018;92:1434–60. - PubMed
-
- Dybul M A grand convergence and a historic opportunity. The Lancet. 2013. Dec 7;382(9908):e38–9. - PubMed
-
- Horton R Offline: Can one turn an aspiration into reality? The Lancet. 2015. Feb 7;385(9967):492.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

