Exploration of Cryptic Pockets Using Enhanced Sampling Along Normal Modes: A Case Study of KRAS G12D
- PMID: 39419500
- PMCID: PMC11558672
- DOI: 10.1021/acs.jcim.4c01435
Exploration of Cryptic Pockets Using Enhanced Sampling Along Normal Modes: A Case Study of KRAS G12D
Abstract
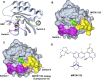
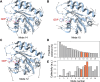
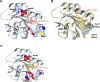
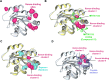
Identification of cryptic pockets has the potential to open new therapeutic opportunities by discovering ligand binding sites that remain hidden in static apo structures of a target protein. Moreover, allosteric cryptic pockets can become valuable for designing target-selective ligands when the natural ligand binding sites are conserved in variants of a protein. For example, before an allosteric cryptic pocket was discovered, KRAS was considered undruggable due to its smooth surface and conservation of the GDP/GTP binding pocket across the wild type and oncogenic isoforms. Recent identification of the Switch-II cryptic pocket in the KRASG12C mutant and FDA approval of anticancer drugs targeting this site underscores the importance of cryptic pockets in solving pharmaceutical challenges. Here, we present a newly developed approach for the exploration of cryptic pockets using weighted ensemble molecular dynamics simulations with inherent normal modes as progress coordinates applied to the wild type KRAS and the G12D mutant. We performed extensive all-atomic simulations (>400 μs) with and without several cosolvents (xenon, ethanol, benzene), and analyzed trajectories using three distinct methods to search for potential binding pockets. These methods have been applied as a proof-of-concept to KRAS and have shown they can predict known cryptic binding sites. Furthermore, we performed ligand-binding simulations of a known inhibitor (MRTX1133) to shed light on the nature of cryptic pockets in KRASG12D and the role of conformational selection vs induced-fit mechanism in the formation of these cryptic pockets.
Conflict of interest statement
The authors declare the following competing financial interest(s): N.V., S.Z., J.P.T, L.A.P, A.D., J.X., A.N., A.G.S., and D.N.L. are current or former employees of OpenEye, Cadence Molecular Sciences. A.M., Y.A.A, and A.K. are former employees of Black Diamond Therapeutics. J.D.L. is a former employee of Mirati Therapeutics, Inc.
Figures

References
-
- Ṡledź P.; Stubbs C. J.; Lang S.; Yang Y.; McKenzie G. J.; Venkitaraman A. R.; Hyvönen M.; Abell C. From Crystal Packing to Molecular Recognition: Prediction and Discovery of a Binding Site on the Surface of Polo-Like Kinase 1. Angew. Chem., Int. Ed. 2011, 50 (17), 4003–4006. 10.1002/anie.201008019. - DOI - PMC - PubMed
-
- Ketcham J. M.; Haling J.; Khare S.; Bowcut V.; Briere D. M.; Burns A. C.; Gunn R. J.; Ivetac A.; Kuehler J.; Kulyk S.; Laguer J.; Lawson J. D.; Moya K.; Nguyen N.; Rahbaek L.; Saechao B.; Smith C. R.; Sudhakar N.; Thomas N. C.; Vegar L.; Vanderpool D.; Wang X.; Yan L.; Olson P.; Christensen J. G.; Marx M. A. Design and Discovery of MRTX0902, a Potent, Selective, Brain-Penetrant, and Orally Bioavailable Inhibitor of the SOS1: KRAS Protein–Protein Interaction. J. Med. Chem. 2022, 65 (14), 9678–9690. 10.1021/acs.jmedchem.2c00741. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

