Exploring P2X7 receptor antagonism as a therapeutic target for neuroprotection in an hiPSC motor neuron model
- PMID: 39419765
- PMCID: PMC11631223
- DOI: 10.1093/stcltm/szae074
Exploring P2X7 receptor antagonism as a therapeutic target for neuroprotection in an hiPSC motor neuron model
Abstract
ATP is present in negligible concentrations in the interstitium of healthy tissues but accumulates to significantly higher concentrations in an inflammatory microenvironment. ATP binds to 2 categories of purine receptors on the surface of cells, the ionotropic P2X receptors and metabotropic P2Y receptors. Included in the family of ionotropic purine receptors is P2X7 (P2X7R), a non-specific cation channel with unique functional and structural properties that suggest it has distinct roles in pathological conditions marked by increased extracellular ATP. The role of P2X7R has previously been explored in microglia and astrocytes within the context of neuroinflammation, however the presence of P2X7R on human motor neurons and its potential role in neurodegenerative diseases has not been the focus of the current literature. We leveraged the use of human iPSC-derived spinal motor neurons (hiPSC-MN) as well as human and rodent tissue to demonstrate the expression of P2X7R on motor neurons. We extend this observation to demonstrate that these receptors are functionally active on hiPSC-MN and that ATP can directly induce death via P2X7R activation in a dose dependent manner. Finally, using a highly specific P2X7R blocker, we demonstrate how modulation of P2X7R activation on motor neurons is neuroprotective and could provide a unique pharmacologic target for ATP-induced MN death that is distinct from the role of ATP as a modulator of neuroinflammation.
Keywords: ALS; ATP; P2X7; hiPSC; purinergic signaling.
© The Author(s) 2024. Published by Oxford University Press.
Conflict of interest statement
A.E.J and N.J.M are unpaid collaborators with Janssen Pharmaceuticals. The other authors declared no potential conflicts of interest.
Figures

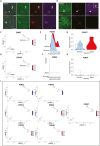

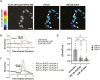
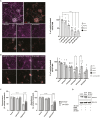
References
-
- Cotrina ML, Nedergaard M.. Physiological and pathological functions of P2X7 receptor in the spinal cord. Purinergic Signal. 2009;5:223-232. https://doi.org/10.1007/s11302-009-9138-2 - DOI - PMC - PubMed
-
- Davalos D, Grutzendler J, Yang G, et al.ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci. 2005;8:752-758. https://doi.org/10.1038/nn1472 - DOI - PubMed
-
- Burnstock G. Introduction to purinergic signalling in the brain. Adv Exp Med Biol. 2020;1202:1-12. https://doi.org/10.1007/978-3-030-30651-9_1 - DOI - PubMed
-
- Burnstock G. The past, present and future of purine nucleotides as signalling molecules. Neuropharmacology. 1997;36:1127-1139. https://doi.org/10.1016/s0028-3908(97)00125-1 - DOI - PubMed
-
- Butt AM. ATP: a ubiquitous gliotransmitter integrating neuron-glial networks. Semin Cell Dev Biol. 2011;22:205-213. https://doi.org/10.1016/j.semcdb.2011.02.023 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

